1. Composition of electrolyte
The basic functions of electrolyte:
It transfers lithium ions between the positive and negative electrodes, but insulates electrons to ensure that the battery can be charged and discharged smoothly.
Ideal electrolyte requirements:
1) It is an excellent conductor for lithium ions and an insulator for electrons;
2) Except for the migration of lithium ions, no other side reactions occur on the electrode surface;
3) Do not react with other battery components;
4) Good chemical stability; safe and environmentally friendly;
The composition of the electrolyte:The composition of lithium-ion battery electrolyte mainly includes organic solvents , lithium salts and additives .2. Organic solvents
Characteristics of an ideal solvent:- High dielectric constant and low viscosity;
- It has a sufficiently high solubility for lithium salts to ensure high conductivity;
- High boiling point and low melting point;
- Good chemical stability; Good electrochemical stability;
- Safety and environmental compatibility; low cost;
The organic solvents used in electrolytes are mainly the following categories: carbonates, acid esters, ether organic solvents, and sulfur-containing organic solvents.2.1 Commonly used carbonate solvents are as follows:Carbonate solvents can be divided into cyclic carbonates and chain carbonates according to their structure . Cyclic carbonate solvents have extremely high dielectric constants, but also have high viscosity; chain carbonate solvents have low dielectric constants, but also low viscosity.Characteristics of carbonate solvents:Carbonate solvents have extremely high dielectric constants;Good electrochemical stability and high oxidation potential;Good compatibility with graphite negative electrode, especially EC can form a good SEI film on the surface of graphite electrode;The mixed use of cyclic carbonate and chain carbonate can meet the requirements of lithium battery working temperature, conductivity and other aspects;Green and environmentally friendly, low cost;2.2 New solvent - carboxylic acid ester:2.3 New solvent - sulfite:3. Lithium salt
Soluble in organic solvents and the solution has high conductivity;The anion has high oxidation and reduction stability;Good chemical stability; Good electrochemical stability;Good safety, environmental friendliness and low cost;Lithium salts can be divided into inorganic lithium salts and organic lithium salts according to the different anions ;3.1 Common inorganic lithium salts, as shown in the following table
3.2 Common organic lithium salts, as shown in the following table
Average ion mobility: LiBF 4 > LiClO 4 > LiPF 6 > LiAsF 6 > LiTf > LiImideDissociation constant: LiTf < LiBF 4 < LiClO 4 < LiPF 6 < LiAsF 6 < LiImideLiPF 6 has a higher conductivity;3.3 Advantages and Disadvantages of Lithium SaltsAdvantages of LiPF6: high conductivity; good electrochemical stability; effective passivation of aluminum foil; good compatibility with graphite negative electrode; low cost.Due to the weak association ability of PF6-, the conductivity of the formed LiPF6 electrolyte is higher than that of other inorganic lithium salts. In addition, it has strong electrochemical stability, the stable voltage of the cathode is 5.1V, which is much higher than the 4.2V required by lithium-ion batteries, and it does not corrode the aluminum current collector. Its comprehensive performance is better than other lithium salts.Disadvantages of other lithium salts:
4. Additives
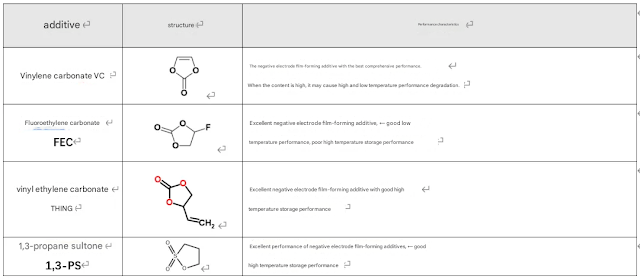
In an electrolyte composed of high-purity solvent and solute, a small amount of additive with specific functions is introduced to significantly improve the performance of the electrolyte in certain aspectsBasic requirements for additives :Small dosage, significant effect.It has no obvious side effects on battery performance and does not react with other components of the battery;The price is relatively low; no toxicity or less toxicity.Additives are classified by function :SEI film-forming additives; anti-overcharge additives; flame retardant additives; electrolyte stabilizers; other additives.4.1 Film-forming additivesFunction: It can be reduced to SEI film on the negative electrode surface before solvent molecules, preventing further decomposition of solvent molecules.Common negative electrode film-forming additives:Among them, VC is the most representative negative electrode film-forming additive.4.2 Anti-overcharge additivesFunction: When the battery is overcharged, the current is blocked in a certain way, thereby improving the safety of the battery.(1) Electropolymerization additives: When overcharged, the additives oxidize and polymerize on the surface of the positive electrode , or produce a large amount of gas, causing the CID to operate and cut off the current, or the oxidation products cover the surface of the positive electrode, resulting in an increase in resistance and a decrease in current, thereby achieving safety protection.
(2) Redox Shuttle AdditivesAdd a suitable redox pair to the electrolyte . When the charging voltage exceeds the normal charge and discharge voltage of the battery, the additive is oxidized at the positive electrode, the oxidation product diffuses to the negative electrode and is reduced, and the reduction product diffuses to the positive electrode and is oxidized. The whole process is repeated until the battery is overcharged.4.3 Flame retardant additivesSince lithium-ion batteries use flammable organic solvents, they may explode and burn if abused. Adding flame retardant additives to the electrolyte can turn flammable organic electrolytes into flame-retardant or non-flammable electrolytes, reduce the heat release value and self-heating rate of the battery, and increase the stability of the electrolyte itself. Currently, most flame retardant additives used in electrolytes are organic phosphorus compounds, organic halides, phosphorus-halogen or phosphorus-nitrogen composite organic compounds.
4.4 Electrolyte StabilizerLiPF6 has poor thermal stability. The PF5 produced by decomposition is a strong Lewis acid that can react with the lone pair of electrons of oxygen atoms in solvent molecules, thereby causing the solvent molecules to decompose and even destroy the SEI film. Electrolyte stabilizers are generally Lewis bases that can form complexes with PF5, thereby improving the stability of the electrolyte.Other additives include water removal or HF additives, wetting promoters , conductive additives, additives to improve low temperature performance, etc.Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials, electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email: contact@canrd.com Phone/Wechat/WhatsApp: +86 19867737979
Canrd Official Web Canrd Company Vedio Canrd Company profile
Website : www.canrud.com

















No comments:
Post a Comment