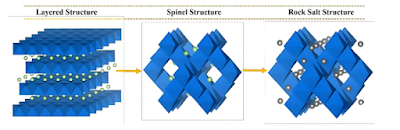
Lithium cobalt oxide is the first commercialized cathode material for lithium-ion batteries. Its theoretical gram capacity after complete delithiation is 274 mAh/g, its true density is as high as 5.1 g/cm 3 , and its actual compacted density can reach 4.2 g/cm 3. It has an extremely high volume energy density (the advantage is prominent under high voltage) and is still the most widely used cathode material for consumer batteries.In fact, lithium cobalt oxide has three crystal structures, namely high-temperature phase HT- LiCo O 2 , low-temperature phase LT - LiCo O 2 , and rock salt phase LiCo O 2. Among them, the synthesis temperature of low-temperature phase lithium cobalt oxide is relatively low, and the crystal structure characteristics are between the layered structure and the spinel structure. The Li layer contains about 25% Co atoms, and the Co layer contains about 25% Li atoms. The bulk density is low and the electrochemical performance is poor. It is rarely used as a commercial positive electrode material. The structure of rock salt phase lithium cobalt oxide is highly disordered, and Li and Co are randomly arranged inside the crystal without obvious rules.
Brief Introduction to the Three Crystal Structures of Lithium Cobalt Oxide
Currently, lithium cobalt oxide commonly refers to the high-temperature phase HT-LiCo O 2 , which belongs to the α-NaFe O 2 layered structure hexagonal crystal system, with a space group of R-3m. Co atoms and adjacent O atoms form Co O 6 octahedrons through covalent bonds, and Li atoms and adjacent O atoms form Li O 6 octahedrons through ionic bonds . Li + and Co 3+ are alternately arranged in the skeleton structure formed by O 2- , forming an "-O-Li-O-Co- O-Li-O-Co- " arrangement structure. Since the Co-O bond force is stronger than the Li-O bond, it helps Li + to escape and embed in the Co O 2 layer during the charge and discharge process . The layered structure of lithium cobalt oxide is not easy to collapse, thereby ensuring that the material has good cycle stability.
Crystal structure model of lithium cobalt oxide
Higher energy density is the unremitting pursuit of lithium-ion batteries. Increasing the upper limit voltage of charging can make lithium cobalt oxide release more Li + to participate in the electrochemical reaction, thereby improving the discharge capacity and discharge platform of the whole battery. For example, increasing the upper limit voltage of lithium cobalt oxide from 4.2V to 4.45V, the discharge capacity is increased from 140mAh/g to 180mAh/g (increased by about 28.6%), and the discharge platform is increased from 3.70V to 3.87V (increased by about 4.6%). Therefore, increasing the upper limit voltage of lithium cobalt oxide charging is one of the most effective ways to increase the energy density of batteries.
Specific capacity and discharge platform corresponding to different upper charging voltage limits of lithium cobalt oxide
However, increasing the upper limit of charging voltage (excessive lithium removal) will bring a series of problems, such as material phase change, interface side reactions, cobalt metal dissolution, oxygen evolution, etc., which will lead to rapid attenuation of material performance, especially cycle performance. The surface reaction activity of lithium cobalt oxide is higher than that of the bulk phase, and the charging process on its surface includes the following reaction steps:
Step 1: Li + is preferentially released from the surface of lithium cobalt oxide ;
Step 2: After Li + is released, the O atoms lose the cation barrier and repel each other, and the surface structure begins to become unstable;
Step 3: Li + is continuously released, and the lattice oxygen activity at the surface increases to a certain level, causing oxygen evolution;
Step 4: After oxygen evolution occurs, the stability of the Co atoms on the surface deteriorates and dissolves;
Step 5: The high-valent element Co 4+ simultaneously oxidizes the electrolyte, directly participates in the chemical reaction and dissolves into the electrolyte.
Capacity decay mechanism of lithium cobalt oxide cathode
As shown in the figure below, during the charging process, as Li + is removed from lithium cobalt oxide, the crystal structure of the material changes, resulting in irreversible damage to the material structure , and it is difficult for Li + to be reversibly deintercalated, resulting in capacity loss. During the charging process of lithium cobalt oxide, there are two phase transitions to the monoclinic system. When charged to 4.2V, the amount of Li + removed is about 50%. At this time, lithium cobalt oxide changes from hexagonal system (H-2 phase) to monoclinic system (M phase). After Li + is removed, the adjacent O atomic layer expands the c-axis of the material unit cell by about 2.3% due to the electrostatic repulsion force greater than the Li-O electrostatic attraction. When lithium cobalt oxide is charged to above 4.5V, lithium cobalt oxide changes from hexagonal system (O3 phase) to monoclinic system (O1 phase), and the c-axis of the material unit cell expands by about 2.6%, which is 13% higher than that at 4.2V. The huge unit cell volume change easily leads to the collapse of the layered structure of the material, resulting in particle cracks or even pulverization. Some Li + cannot be reintercalated into the CoO2 layer , which ultimately leads to a rapid decay of the cycle capacity retention rate.
LixCoO2Differential capacity of (0≤x≤1) as a function of Li + concentration
The second problem caused by the increase in the upper voltage limit of lithium cobalt oxide is the increase in interfacial side reactions. The electrochemical stability of the electrolyte deteriorates under high voltage, and it is easy to decompose and produce HF, which corrodes the SEI film and affects the deintercalation of Li + . Secondly, lithium cobalt oxide in a highly delithiated state contains highly oxidizingCo 4+ .The electrolyte is oxidized on the surface of lithium cobalt oxide to produce by-products, thereby increasing the interfacial impedance and ultimately leading to accelerated capacity decay.
The reaction mechanism is as follows:
LiPF6→LiF(s)+PF5
H 2 O (trace amount of water) + PF 5 → PO F 3 + 2HF
The electrolyte lithium salt LiPF6 is an extremely unstable substance. It decomposes rapidly under high voltage to produce LiF insoluble in the electrolyte and highly reactive Lewis acid PF5 , which then reacts with trace water in the electrolyte to generate HF. Studies have shown that terminal hydroxyl LiCoO2 is highly reactive to HF, thereby generating H2O and LiF precipitation, which adhere to the electrode surface and hinder the migration of Li + between the positive electrode material and the electrolyte interface, resulting in battery capacity decay.
The third problem is the dissolution of cobalt metal and the evolution of oxygen. As Li + is continuously released, the activity of Co and O on the surface of lithium cobalt oxide is further enhanced. When the lattice oxygen activity increases to a certain level, it will escape in the form of O2 . After the gas escapes, the stability of Co atoms deteriorates, and this process is accompanied by Co dissolution.
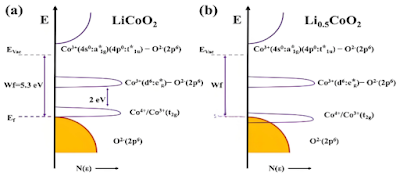
Among common transition metal elements, compared with Mn, Ni and other elements, Co and O have the strongest interaction, and the overlap of their electronic orbits is most obvious. As the upper limit of charging voltage increases, the electrons lost by the positive electrode material gradually increase. When the top electrons of the valence band of Co element higher than the 2p orbital of O element are exhausted, further charge compensation will occur in the overlapping area of t2g orbital of Co and 2p orbital of O. That is, the charge compensation in this area is no longer provided by Co element independently, but the anion O element will also participate. From the electronic band structure diagram of lithium cobalt oxide in the figure below, it can be seen that when lithium cobalt oxide is not delithiated, the Co 4+/3+ (t2g ) energy band and the O 2- (2p ) energy band are separated. When half of the Li + in the lithium cobalt oxide is delithiated, the Co 4+/3+ (t2g ) energy band and the O 2- (2p ) energy band partially overlap, and the lattice O participates in charge compensation. Further delithiation will lead to O 2 escape and Co dissolution, the stability of the material skeleton structure will deteriorate, and ultimately lead to the attenuation of electrochemical performance and safety performance.
Electronic band structure of lithium cobalt oxide
Due to its own material structure, lithium cobalt oxide is prone to irreversible phase change under high voltage (excessive lithium removal), unstable layered structure, accompanied by crystal plane slip, atomic rearrangement leading to drastic changes in unit cell parameters, dislocation of grain boundaries, surface stress changes causing particle rupture, electrolyte oxidation and decomposition producing side reactions, oxygen escape, cobalt metal dissolution and other problems leading to battery performance failure. At present, lithium cobalt oxide is mainly modified by surface coating and bulk doping to improve its stability under high voltage.
Common modification ideas for lithium cobalt oxide materials
Surface coating mainly changes the physical and chemical state of the surface of lithium cobalt oxide, and stabilizes its material morphology and crystal structure to a certain extent. Through surface coating treatment, a "barrier" can be built on the surface of lithium cobalt oxide material to stabilize the interface between active materials and electrolyte, prevent HF from corroding electrode materials, and inhibit interface side reactions such as cobalt dissolution and electrolyte oxidation. Bulk doping mainly improves the structural stability of lithium cobalt oxide and improves the intrinsic properties of lithium cobalt oxide . After the doping elements enter the Li, Co, and O positions , due to the differences in the doping elements, the atomic state of the sites they occupy will be changed, thereby changing the overall atomic arrangement and electron cloud distribution of the material, and then affecting the material performance. It is an important method to overcome the defects of the material itself and optimize the material performance.
The problems of cobalt dissolution, electrolyte oxidation decomposition and material phase change of lithium cobalt oxide at 4.2V can be effectively solved by surface coating. As for the material phase change problem and oxygen loss problem above 4.5V, multi-element synergistic doping is a more effective modification strategy (such as main group element doping: Na, Mg, Ca, Al, Cu, etc., transition metal element doping: Ni, Mg, Ti, Zn, Cr, Fe, etc., non-metallic element doping: B, P, Si, F, etc., rare earth element doping: Nd, La, etc. ) , but the improvement of structural stability by bulk doping is not the only factor affecting the performance of high-voltage lithium cobalt oxide. It needs to be combined with surface coating to inhibit interface side reactions, cobalt dissolution and oxygen escape. Therefore, a single method may not be able to take into account both material structure and interface problems . The effective combination of the two modification methods is an important technical means .
Surface coating technology mainly includes four categories:
1) Electronic conductor coating;
2) Ion conductor coating;3) Electronic and ionic dual conductor coating;
4) Electronic and ion double insulation coating.
The electronic conductor coating mainly includes carbon (C) coating and coatings of other compounds with high electronic conductivity. However, due to the semiconductor properties of lithium cobalt oxide (LiCoO₂) itself, and the inevitable generation of Li vacancies during the synthesis process, its electronic conductivity is extremely excellent. It is currently the material with the highest electronic conductivity among the cathode materials for lithium-ion batteries, so no special improvements are needed. In addition, at high temperatures, carbon can reduce lithium cobalt oxide to CoO and Co₃O₄, making it difficult to apply from a technical perspective. Since LFP (Lithium Iron Phosphate) has lower electronic conductivity and ionic conductivity, the application of carbon coating on LFP has been the most successful, significantly improving its electrochemical performance.
Performance comparison of common positive electrode materials
Ion conductor coating is to coat the surface of lithium cobalt oxide with a layer of material with high ionic conductivity. Since solid electrolyte materials usually have high ionic conductivity and wide electrochemical window, they are often used as ion conductor coating materials. For example, Li 1.5 Al 0.5 Ti 1.5 (PO 4 ) 3 ( LATP for short) coated on the surface of lithium cobalt oxide can greatly improve the cycle stability at 4.5V, and also improve the material rate performance to a certain extent. However, electronically insulating and heavy solid electrolyte coating may also lead to problems such as increased internal resistance of the material and reduced specific capacity. Therefore, ultra-thin continuous solid electrolyte coating should be the main technical direction.
Comparison of lithium cobalt oxide performance before and after LATP coating
Electronic and ionic dual-conductive coatings refer to materials that can conduct both electrons and lithium ions. These materials can typically also be used independently as electrode active materials, such as LiMn₂O₄, LiFePO₄, Li₂TiO₃, Li₄Ti₅O₁₂, and LiNbO₃. The earliest reported electronic and ionic dual-conductive coating material was lithium manganese oxide, which was initially applied to 4.3V lithium cobalt oxide materials, achieving breakthrough progress. Subsequently, lithium iron phosphate (LiFePO₄), with a higher specific capacity, became a more suitable choice. Its excellent stability also protects the cathode surface from electrolyte corrosion. At 4.2V, LFP coating significantly improves cycling stability and high-temperature performance, while at higher voltages (such as above 4.5V), lithium niobate (LiNbO₃) coated lithium cobalt oxide demonstrates even better cycling performance.
HRTEM image of LiNbO3 - coated lithium cobalt oxide (three-phase mixed coating)
It seems that electronic and ionic dual insulating coating will not have any other beneficial effects on battery performance except physical barrier. However, this is not the case. Currently, the most common and ideal coating method is electronic and ionic dual insulating coating, such as oxides ( Al2O3,MgO),fluorides, and phosphides.
The academic community has a consensus on the improvement of material performance by oxide coating: oxide coating can achieve physical barrier and chemical stability, and alleviate Co dissolution. At the same time, the coated oxide has a dual oxidation property, which can react with both acid and alkali, and can consume corrosive substances such as HF in the battery system, and improve the interface stability of the active material. However, there are still two controversial points about oxide coating: one is the continuity of the coating. Unlike the coating of electronic and ionic dual conductors, the oxide itself is not conductive. A completely dense coating or a coating with a thickness exceeding the electron tunneling distance will cause the electrochemical performance of the active material to decrease. How to control the thickness and continuity of the coating is a major difficulty; the second is the form in which the coating exists, whether it exists in the form of an inorganic substance or forms a solid solution with lithium cobalt oxide. There is still controversy.
Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials, electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email: contact@canrd.com Phone/Wechat/WhatsApp: +86 19867737979
Canrd Official Web Canrd Company Vedio Canrd Company profile
Website : www.canrud.com


















No comments:
Post a Comment