Interfacial friction makes the vertical structure of lithium metal batteries
summary
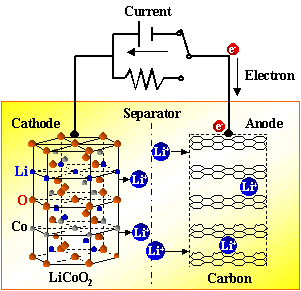
A practical high-energy-density lithium metal battery requires a free-standing lithium metal anode with a thickness of less than 20 μm, but it is difficult to achieve large-scale processing of thin layers and free-standing structures due to the low melting point and strong diffusion creep effect of lithium metal. In this study, a free-standing lithium chips with a thickness of 5 to 50 μm was formed on the lithium metal surface by mechanical rolling, which was determined by the in-situ tribochemical reaction between lithium and zinc dialkyl dithiophosphate (ZDDP). A layer of organic/inorganic hybrid interface (about 450 nm) was formed on the lithium metal surface with extremely high hardness (0.84 GPa) and Young's modulus (25.90 GPa), which not only enables scalable processing of lithium chips, but also realizes dendrite-free lithium metal anode by inhibiting dendrite growth. The rolled lithium anode has a long cycle life and high-rate cycling stability ( more than 1700 cycles at 25°C even at current densities of 18.0 mA cm −2 and 1.5 mA cm −2 ). This work provides a scalable tribological design approach for producing practical thin free-standing lithium metal anodes.