Pre-lithiation of silicon-based anodePreface
With the development of society and the advancement of science and technology, energy consumption is increasing day by day, environmental pollution is also becoming increasingly serious, and has seriously threatened the future survival of mankind. Therefore, it is urgent to develop clean and environmentally friendly renewable energy. However, most renewable energy sources such as wind energy and solar energy are unstable and intermittent, while batteries can directly convert chemical energy into electrical energy, which is not only stable but also has high energy conversion efficiency, which can effectively alleviate the energy pressure we are facing now. Among them, lithium-ion batteries have been rapidly developed due to their advantages such as high energy density, long cycle life, and environmental friendliness, and are widely used in the fields of electronic products and electric vehicles.
The negative electrode materials of lithium-ion batteries mainly include alloys, carbon-based, Li4 Ti5 O12 and transition metal compound materials. Among them, carbon-based graphite negative electrode materials are the most commonly used, but this material has a low specific capacity (372mA·h·g-1) and a large irreversible capacity loss, which makes it difficult for lithium-ion batteries to meet the performance requirements and there is little room for development. Silicon has a high specific capacity (4200mA·h·g-1) and abundant resources, and it is expected to replace graphite as one of the most promising negative electrode materials for batteries.
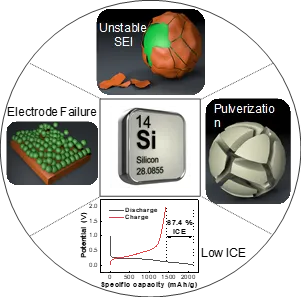
However, silicon has a large volume expansion (300%) and huge capacity attenuation during the lithium removal/insertion process, which directly leads to the instability of the solid electrolyte interface film (SEI film). A stable SEI film is the main factor in extending the battery cycle life. In addition, the formation and destruction of the SEI film during the charging and discharging process of lithium-ion batteries will continuously consume lithium ions, resulting in low initial coulombic efficiency and short cycle life of lithium-ion batteries.
To address the above problems, the most effective solution at present is to use pre-lithiation technology, which is to add a small amount of lithium consumed in excess in the lithium source equilibrium reaction before the electrode is officially charged and discharged, to supplement the consumption of cathode lithium in the side reactions and SEI film formation process, so as to improve the initial coulombic efficiency, extend the battery cycle life, alleviate volume expansion to a certain extent, and improve the overall performance of lithium-ion batteries.
Pre-lithiation technology types and research practices
1: Pre-lithiation of stable lithium metal powder
Stabilized lithium metal powder (SLMP) is a core-shell particle powder produced by FMC Lithium, USA. It is a pre-lithiation agent that can be put into commercial production and application. The average particle size of SLMP is 10~50μm, and it is composed of about 97% lithium metal powder and 3% Li2CO3 by mass. Among them, Li2CO3 is evenly coated on the surface of lithium particles as a protective film, which can effectively prevent the occurrence of harmful side reactions. This unique composition structure requires SLMP to apply pressure during the pre-lithiation process to break the Li2CO3 coated on the surface of lithium metal, so that lithium metal can be effectively utilized.
The research team experimentally studied the performance of SLMP in pre-lithiation. The results showed that when the external pressure was 6MPa, the protective film Li2CO3 was broken, and the metallic lithium and the electrode material could fully contact each other. When the usage of metallic lithium powder was 3g/m2, the utilization efficiency of metallic lithium powder was 56%, and the first discharge efficiency exceeded 18%. It had almost no effect on the initial charging capacity of the electrode, but the cycle performance of the electrode was improved.
There are also research teams that mix toluene solution with SLMP, then drop the mixture onto the Si/CNT anode, wait for the toluene solvent to evaporate, and then perform pressure activation. After 40-50 hours, the metal lithium powder in the SLMP is embedded in the Si/CNT composite anode to achieve pre-lithiation. The results show that the pre-lithiation treatment of the Si/CNT anode can eliminate the 20%-40% irreversible capacity loss in the first cycle; the full battery assembled with the Si/CNT anode and the lithium nickel cobalt aluminum oxide (NCA) cathode maintains 93% of the initial capacity at 100% discharge depth, and can achieve more than 1,000 cycles of stability at 20% discharge depth.
A research team in the industry mixed SLMP with a hexane solution to form a suspension, and used an adjustable pipette to drop the SLMP/hexane suspension onto the SiO negative electrode, and then pressure activated it after the hexane solvent evaporated completely. Before pressure activation, SiO was subjected to disproportionation reaction and asphalt pyrolysis reaction to obtain a micron-sized d-SiO/G/C negative electrode composite material (the composite material has good cycle performance and conductivity, and the initial reversible capacity can reach 905mA·h·g-1). The results show that the electrochemical performance of lithium-ion batteries is better when SLMP is coated on the surface of the d-SiO/G/C composite material for pre-lithiation. The initial coulombic efficiency of the d-SiO/G/C negative electrode is increased from 68.1% to 98.5%, and the excellent cycle retention rate of 95% is still maintained after 200 cycles.
Figure 1 Preparation process of d-SiO/G/C composite material, SLMP pre-lithiation process, negative electrode Coulombic efficiency and cycle performanceAs shown in Figure 1, controlling the amount of SLMP can effectively improve the first coulombic efficiency of the negative electrode material, while offsetting the irreversible loss of lithium ions in the formation of the SEI film and promoting the formation of the SEI film. SLMP can alleviate the irreversible capacity loss of SiO, and the metal lithium powder therein participates in the irreversible reaction to generate lithium silicon oxide, thereby improving the preparation process of lithium ion d-SiO/G/C composite materials, the SLMP pre-lithiation process, the coulombic efficiency and cycle performance of the negative electrode, and improving the cycle stability of the battery. The pre-lithiation scheme of the d-SiO/G/C negative electrode composite material is novel and can be used as a reference. A research team has also developed a solution treatment method to disperse SLMP in a xylene binder solution of SBR/polystyrene, fully mix to form a uniform and stable SLMP slurry , and then coat the SLMP slurry on the dry negative electrode surface. By controlling the applied calendering pressure to activate SLMP, a uniform and scalable SLMP coating is obtained on the negative electrode surface. The electrode material prepared by this method has achieved ideal electrochemical performance in both silicon monoxide SiO/NMC full cells and SiO half cells.In addition, other research teams used the SBR-PVDF composite adhesive system to directly mix SLMP into the electrode slurry. After coating, the first coulomb efficiency increased from 90.6% to 96.2%, and the pre-lithiation effect was good. The above studies show that SLMP pre-lithiation can provide an effective lithium source for silicon-based negative electrodes, which is conducive to the formation of a stable SEI film, thereby improving the electrochemical performance of the battery; by controlling the amount of SLMP, the degree of pre-lithiation can be easily controlled, which can not only solve the problem of insufficient pre-lithiation causing the first coulomb efficiency to be unable to improve, but also prevent excessive lithium supplementation from causing lithium deposition and waste of resources. SLMP pre-lithiation technology is the most commonly used in production practice, but SLMP is expensive, and it is easy to cause dust and pollute the environment during the pre-lithiation process, so this technology needs to be further improved.2: Electrochemical pre-lithiationThe direct contact method is that the silicon-based electrode and the lithium material are in direct contact, electrons are transferred from the silicon-based electrode, and lithium ions diffuse into the interior of the electrode to complete the pre-lithiation operation.The research team placed the Si/CNT electrode in contact with the lithium foil for 30 minutes, then dripped a small amount of battery electrolyte solution between the Si/CNT electrode and the lithium foil to embed lithium ions through pre-lithiation. The results showed that the open circuit voltage of the Si/CNT electrode dropped to 0.47V after electrochemical pre-lithiation; the first discharge capacity was 2188mA·h·g-1, much smaller than the original electrode of 4038mA·h·g-1, but the first charge capacity was 1850mA·h·g-1; the first coulombic efficiency increased to 102%, much higher than the first coulombic efficiency of the Si/CNT electrode without pre-lithiation (47%), effectively solving the problem of low first coulombic efficiency of Si/CNT composite materials. The research team used a self-discharging pre-lithiation silicon nanowire negative electrode, directly contacted the silicon nanowire with the metal lithium foil, and then placed it in a certain amount of electrolyte, and applied moderate pressure to make the silicon nanowire electrode contact with the lithium foil for pre-lithiation. The results show that after the pre-lithiation, the metal lithium can be reused after being washed with electrolyte solvent, and the utilization rate reaches 100%; SEI film is formed on the surface of the silicon nanowire, the first irreversible capacity loss of the silicon-based negative electrode is reduced, and the energy density of the lithium-ion battery is improved; 50% of the capacity of the silicon nanowire negative electrode can be achieved in 20 minutes, and the actual capacity density can reach 2000mA·h·g-1.The indirect contact method is that the silicon-based electrode is not in direct contact with the lithium material, and the pre-lithiation operation is completed through a certain medium or a certain process. The research team monitors the voltage of the c-SiOx electrode and forms a short-circuit state under the action of the potential difference by accurately matching the short-circuit time. The c-SiOx electrode is spontaneously pre-lithiated, and the degree of pre-lithiation can be adjusted, as shown in Figure 2.
Figure 2 Schematic diagram of c-SiOx electrode pre-lithiation and industrializationThis is a precise and scalable external short-circuit pre-lithiation technology. The structure of SiOx is stable during the entire cycle. The presence of a diaphragm prevents direct contact between the metal lithium and the c-SiOx electrode, which can avoid the deposition of metal lithium and promote the formation of a stable SEI film, allowing the initial coulombic efficiency to be as high as 94.9%. During the pre-lithiation process, moisture and oxygen have a great influence on the stability of metal lithium, in order to further improve the safety of lithium supplementation.Another research team has developed a three-layer structure of active material/polymer/lithium negative electrode, using the polymer layer to protect metallic lithium from air and moisture. In the experiment, the silicon-based active material was first coated on the polymer PMMA, and then the electrolyte solution was added to the device. PMMA will gradually dissolve when it comes into contact with the battery electrolyte solution, causing the electrode material to come into contact with lithium to form a lithiated negative electrode. The results show that the SiNPs negative electrode achieved an initial coulomb efficiency of up to 100%, and showed higher cycle stability after 100 cycles, reaching 1456mA·h·g-1 (809mA·h·g-1 after 100 cycles). This pre-lithiation technology can control the degree of pre-lithiation by changing the thickness of the lithium layer, and the operation is relatively convenient, but it has more stringent requirements on the device conditions, making it difficult to achieve large-scale application.Lithium metal is relatively expensive. In order to reduce the cost of pre-lithiation, lithium-containing electrolyte solutions can be used through electrochemical reactions. A research team has designed a new green and environmentally friendly electrolytic cell to pre-lithiate silicon electrodes, in which the copper pitting anode half-cell uses aqueous Li2SO4 as the electrolyte solution, and the silicon lithiation cathode half-cell uses a gel polymer as the electrolyte solution. The full battery assembled by them is placed in a LiPF6/EC+DEC solution, and a copper wire is used as the counter electrode. When the silicon-based negative electrode pre-lithiated by this method is assembled into a MnOx/Si full battery, a high specific energy of 349W·h·kg-1 is achieved at a specific power of 20W/kg. Even at a high specific power of 1710W/kg, the specific energy of the entire battery remains at 138W·h·kg-1. It shows that the pre-lithiation technology using electrochemical reactions can make full use of resources, reduce costs, and has high controllability3: Additive pre-lithiationThe additive for pre-lithiation of silicon-based negative electrodes is mainly nano-lithium silicide powder Lix Si. Compared with SLMP, Lix Si additives are smaller in size, with a particle size of only 100~200 nm, which is more conducive to its uniform dispersion in silicon-based negative electrode materials, and Lix Si has little effect on the volume change of the electrode. Therefore, Lix Si additives are widely used in pre-lithiation of silicon-based negative electrodes.The research team used SiO or SiO2 as a precursor and used a metallurgical process to embed uniformly dispersed Lix Si nanoparticles into a highly crystalline Li2O matrix to prepare a Lix Si-Li2O composite material. The results showed that the composite material still had excellent stability in air with a relative humidity of 40%, and could maintain a high capacity of 1240 mA·h·g-1 after being exposed to air for 6 hours. Afterwards, a new silicon-based negative electrode pre-lithiation additive, namely core-shell structured Lix Si-Li2 O nanoparticles, was synthesized by a thermal alloy method, as shown in Figure 3. SiNPs (diameter of about 50nm) and metal lithium foil of a certain stoichiometric ratio were placed in a glove box and mechanically stirred at 600℃ for 6 hours to obtain the Lix Si-Li2O additive. The trace oxygen present in the glove box formed a dense Li2O passivation layer on the surface of Lix Si, which could prevent further oxidation of Lix Si. The capacity retention rate of the Lix Si-Li2 O additive was 91% after being exposed to dry air for 1 day. Using this method for pre-lithiation of silicon-based negative electrodes can reduce the consumption of lithium ions and improve the first coulombic efficiency.Figure 3 Schematic diagram of Li2O coating LixSi process
LixSi additives are sensitive to humid environments and can exist briefly in dry air environments. The research team used the alloying reaction between silicon and metallic lithium to synthesize Lix Si, and synthesized a dense coating layer on the surface of Lix Si particles. The results show that the synthesized Lix Si can be stored for about 5 days in a dry environment and for about 6 hours in relatively humid air; the synthesized Lix Si has good lithium replenishment performance, and as a pre-lithiation additive, it can make the first coulombic efficiency exceed 100%. Therefore, Lix Si additives can be better used in the pre-lithiation treatment of graphite, silicon-based and other negative electrode materials.The research team prepared Si@SiOx by heating silicon nanopowder under air conditions, mixed the prepared Si@SiOx with LiBH4 additives evenly, and then heated them in a sealed autoclave. The Li+ released by the thermal decomposition of LiBH4 reacted with SiOx on the silicon surface to form a uniformly coated Li2 SiO3 layer. The LiBH4 additive can pre-lithiate the Si@SiOx coating and prepare an in-situ formed Li2 SiO3 layer, which can effectively alleviate the volume expansion of the silicon-based negative electrode and improve the electrochemical performance of the battery. The results show that the pre-lithiated Si@SiOx negative electrode material has a first coulombic efficiency of 89.1%, a rate performance of 959 mA·h·g-1 at 30A·g-1, and a capacity retention rate of 3215mA·h·g-1.4: Mechanical pre-lithiationMechanical pre-lithiation is the pre-lithiation of silicon-based negative electrode materials through physical methods such as high-energy ball milling. The research team used a low-temperature mechanical ball milling process to pre-lithiate the synthesized SiNA silicon nanoalloy material, and then mechanically mixed the synthesized silicon nanoalloy material with the lithium-based additive lithium stearate, and placed it in a temperature slightly higher than the melting point of lithium stearate (220°C) for pre-lithiation heat treatment to eliminate the microstructural defects in the form of cracks or voids in the SiNA itself. The results show that the initial charge capacity of the pre-lithiated SiNA reached 997.89mA·h·g-1, and the coulombic efficiency was 89.9%. It showed a relatively high capacity of 710.69mA·h·g-1 during the initial cycle; it showed excellent electrochemical performance and good cycle stability after 250 cycles at a current density of 150mA·h·g-1, with less capacity decay and lower impedance. The industry also uses high-energy mechanical ball milling to prepare lithium-intercalated compounds and study the crystal structure, stability and electrochemical properties of lithium-ion silicon-based negative electrode materials Lix Si. The results show that Li4.4 Si exhibits the best electrochemical performance, with an initial discharge capacity of 3306 mA·h·g-1. At a current density of 358 mA·h·g-1, it maintains a capacity of 2100 mA·h·g-1 after 30 cycles of discharge, and still maintains 1200 mA·h·g-1 after 60 cycles; Li4.4 Si shows good stability at 300~350°C. However, due to the high reaction activity of the lithiated Lix Si, new problems are encountered when preparing electrode materials by mechanical ball milling, so it is necessary to develop efficient and stable preparation technology.
Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials, electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email:contact@canrd.com Phone/Wechat/WhatsApp:+86 19867737979
Canrd Company Vedio/Website:www.canrud.com













No comments:
Post a Comment