Negative electrode binder performance requirements, test methods and failure mechanisms!
Binders have a low mass ratio in the electrode and do not participate in the electrochemical reaction. Their main function is to adhere the active material and the conductive agent to the current collector to keep the electrode intact . Binders affect the formation of the solid electrolyte interface (SEI) , the charge transfer inside the electrode and between the electrode - electrolyte interface , the wetting behavior of the electrode, and the cycle performance and cost of the battery .
This paper briefly introduces the working principle , basic performance requirements , evaluation methods and failure mechanism of binders . The research progress of graphite anode binders for lithium-ion batteries is summarized, and the key issues of silicon anode and its binder design strategy are discussed .
1 Basic properties of adhesives
1.1 Working Mechanism
The essence of adhesive bonding is the interaction between material molecules ( van der Waals force , surface tension, etc. ), chemical bond force ( hydrogen bond , covalent bond , coordination bond, etc. ) and interfacial electrostatic attraction . Figure 1(a) is a schematic diagram of three theories of adhesive bonding . (1) Diffusion theory. (2) Electrostatic theory. (3) Adsorption theory .
1.2 Failure mechanism
The destruction or failure of the binder structure will lead to the destruction of the ion and electron paths and the loss of active materials, thus causing battery capacity decay and safety hazards . The damage mechanism can usually be attributed to three reasons (Figure 1(c)): (1) Contact interface damage : The adhesive strength is insufficient, and the adhesive cannot effectively combine with the adherend, resulting in electrode detachment . (2) Binder rupture: The binder will be as shown in Figure 1(b) . When the stress exceeds the yield strength , the deformation of the polymer is plastic deformation, and the strain cannot be fully recovered after the stress is removed; when the stress exceeds the ultimate strength, the polymer breaks and the binder fails . (3) Adhesive fracture: The adhesive strength and mechanical strength of the binder meet the requirements, but during the battery cycle, the cracking and shedding of the electrode material will also cause the battery capacity to decrease.
1.3 Performance requirements
An ideal binder should have six characteristics: (1) Stable thermal properties to maintain strong bonding over a wide operating temperature range; (2) Good mechanical properties, including tensile strength , elasticity, flexibility, hardness, and bonding strength; (3) Good electrical conductivity and ionic conductivity. (4) Excellent dispersion properties in the solvent to cover and connect the various components of the electrode and prevent uneven aggregation of the slurry; (5) Excellent chemical and electrochemical stability to meet the application requirements of different chemical solvents and different voltage windows; (6) Low cost and good environment to facilitate mass production .
2 Evaluation method of adhesive
2.1 Physical testing methods
(1) Bonding strength test: The peel strength test of the pole piece can be used to directly quantify the bonding strength between the active material and the current collector . This test measures the bonding performance by peeling the pole piece at 90° or 180° . Based on the action mechanism of the adhesive, the surface and morphology of the material determine its interfacial adhesion. Therefore, the bonding strength can be judged by further observing the surface morphology of the material before peeling and the wire drawing of the pole piece surface after peeling by scanning SEM and TEM. The bonding strength of the adhesive is closely related to its chemical structure and functional groups , and can be studied with the help of spectroscopic techniques such as (XPS , FTIR and Raman) .
(2) Mechanical strength test: The mechanical strength and the cause of adhesive failure can be analyzed by studying the stress of the adhesive film under different strains through tensile, shear and fatigue tests .
( 3) Scratch test: Scratch test is an important mechanical testing method. Scratch test is performed on the electrode surface to reveal the mechanical response of the electrode, evaluate the adhesion strength of the sliding surface , and quantify the mechanical properties of the negative electrode prepared based on different binders . The friction coefficient can reflect the composite properties of the electrode and the mechanical properties of the polymer binder . In addition, nanoindentation test can also characterize the hardness of the binder .
(4) Dispersibility: The dispersibility of the slurry affects the subsequent production quality and battery performance of lithium-ion batteries . The influence of the binder on the dispersion of the electrode slurry can be verified by testing the rheological properties of the slurry .
(5) Glass transition temperature ( Tg): When the temperature is lower than Tg , the polymer chains are gradually frozen and begin to crystallize, resulting in the loss of viscoelasticity and interfacial affinity, and the binder loses its ability to adhere . Therefore, the binder should have a lower Tg value .
(6) Thermal stability test: Almost all properties of polymer binders are closely related to thermodynamics. At different temperatures, factors such as thermal stability, diffusivity and expansion rate have an important influence on the electrochemical performance and stability of the electrode. Thermal TGA and DSC can be used to detect the thermal stability of the binder .
2.2 Electrochemical testing
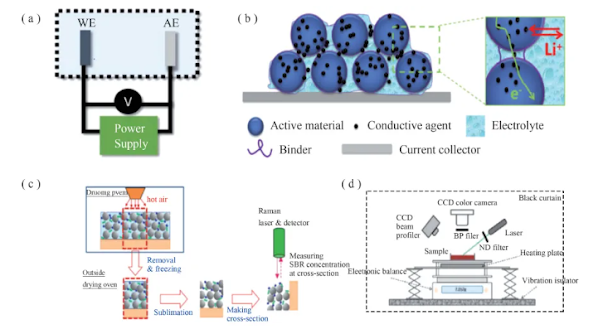
Electrochemical tests mainly observe the electrochemical stability of the binder , the internal resistance of the composite electrode , charge transfer and long-term cycle performance . The electrochemical characterization of battery materials is usually carried out using a two-electrode system consisting of a working electrode (WE, electrode to be analyzed ) and an auxiliary electrode (AE), as shown in Figure 3(a) . Lithium metal has a small overpotential and a stable voltage in a wide electrochemical window, so it is used as an auxiliary electrode.
When studying the specific reaction of WE, the two-electrode system cannot obtain the electrochemical . Therefore, a three-electrode system can be constructed by adding a reference electrode (RE) to the two-electrode battery to obtain detailed information inside the electrode. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) tests can effectively study the electrochemical response of the material within the working voltage window . The electrochemical stability of the polymer binder can be judged by the formation of the decomposition reaction peak on the curve .
2.3 Swelling test
As shown in Figure 3(b), it is generally believed that the binder either covers the active material particles to form an amorphous coating with a thickness of 2 to 35 nm, or becomes fibrous under the action of capillary force to bind the particles. After the battery is filled with electrolyte, the electrolyte diffuses into the electrode pores, and the binder first contacts the electrolyte . The binder should have a high electrolyte wetting ability, improve the interaction with other electrode components, overcome the interface barrier between the electrode components, and promote the efficient transmission of Li +. However, excessive swelling of the binder in the electrolyte will weaken the oxidation resistance of the electrode material, thereby affecting the mechanical strength of the binder, and ultimately increase the battery resistance, resulting in rapid capacity decay . Therefore, the binder should maintain an appropriate swelling rate and strong adhesion to ensure the integrity of the electrode.
Test method: Soak the adhesive film in the electrolyte at room temperature for 48 hours, absorb the surface electrolyte with dust-free paper and weigh it . The initial mass of the adhesive film is recorded as m0 , and the mass after soaking is recorded as m1 . The swelling rate is (m1- m0 )/m0 .
2.4 Binder distribution during drying
Typically, the negative electrode of a lithium-ion battery is obtained by drying a uniform aqueous dispersion of carbon , binder, and additives . Electrode drying is a complex top-down process that includes the diffusion of the binder and the particle sedimentation controlled by the solvent evaporation rate . The uniformity of the binder distribution in the thickness direction affects the performance of the battery, but this parameter is difficult to measure directly. Therefore, researchers often use Raman spectroscopy and real-time fluorescence microscopy to observe electrode evolution and perform model verification analysis.
As shown in Figure 3(c), Hagiwara et al. prepared samples using a drying device that integrates an analytical balance and surface temperature measurement to capture the coating structure in the intermediate stage of drying . Raman spectroscopy was used to quantitatively analyze the binder concentration at different points in the cross- section freeze-dried electrode coating . As shown in Figure 3(d) , Lim et al. used real-time fluorescence microscopy to study the migration of latex particles during electrode drying and qualitatively analyzed the distribution of the binder in the electrode through the time evolution of the fluorescence signal .
2.5 Effects of temperature and humidity on adhesives
The binder needs to have good chemical stability and not react chemically with the electrolyte or other substances . Its stability is related not only to its composition and structure, but also to the chemical environment in which it is located . PVDF is one of the binders with the best chemical stability , but at high temperatures , PVDF easily reacts with metallic lithium and LixC 6 , and swells in the electrolyte , destroying the conductive network and thus deteriorating the battery performance.
In an environment with high pH , PVDF is easy to remove HF to form C=C double bonds . The water in the slurry or the amine in the solvent will attack the C=C double bonds, causing cross-linking and gelation of the slurry, thereby reducing production capacity and deteriorating battery performance .
There are many polar functional groups in the polymer binder molecules, which are easy to absorb moisture. Moisture will react with lithium ions during high-temperature storage to precipitate hydrogen, causing battery swelling . In addition, the film-forming temperature of the binder significantly affects the quality of electrode coating. When the film-forming temperature of the binder is high, the coating quality of the electrode is poor . Therefore , the production process needs to strictly control moisture and temperature to reduce the occurrence of problems in the production process .
3. Binder for graphite negative electrode
Graphite is widely used as anode material in production due to its high conductivity , low cost and good capacity retention . Driven by the strong demand for high-performance lithium-ion batteries, research has now shifted to the development of fast-charging , long-cycle-life , high-power and more environmentally friendly batteries . Despite substantial progress, the development of electric vehicles is still limited by the insufficient battery charging speed . Improving the mass transfer of electrolytes and charge transfer are two key issues that need to be addressed to achieve fast battery charging.
As an important component of the electrode, the binder determines the quality of the SEI and the mechanical and electrochemical connectivity of the electrode. Fast charging often destroys the integrity of the electrode . Commonly used negative electrode binder systems are CMC and SBR, both of which have good rheological and mechanical properties . However, their relatively poor conductivity , low ion migration rate and limited electrolyte permeability significantly affect the thick electrode and high current charge and discharge performance .
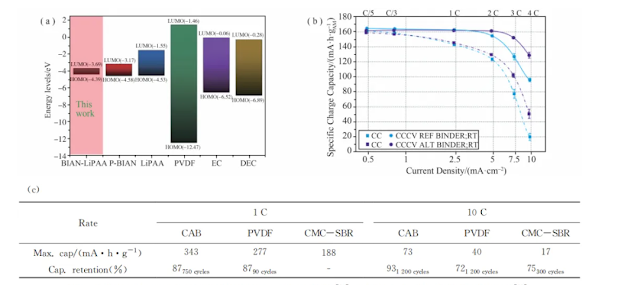
In order to improve the fast charging capability of lithium batteries, Mishra et al. prepared a composite polymer binder BIAN-LiPAA containing Li + to achieve fast charging and better ion diffusion . The low energy level of the lower unoccupied molecular orbital of the BIAN -LiPAA binder reduces the decomposition of the electrolyte, thereby forming a thin and ion-conductive SEI. As shown in Figure 4(a), the composite binder has a low charge transfer resistance and a low lithium insertion activation energy, which significantly improves the diffusion of Li + in graphite .
During fast charging, after 2000 charge-discharge cycles, the capacity retention of BIAN-LiPAA at 10C and 5C was 94.2% and 83.5 %, respectively. Scheck et al. developed a water- based binder system to improve the fast charging capability of graphite anode. As shown in Figure 4(b), the alginate, polyethylene oxide, and polyvinyl pyrrolidone binder system in a 2.5 mA·h/cm2 soft -pack battery showed a more than 150% improvement in charging performance at 4C constant current charging compared to the control binder system composed of CMC and SBR . The improvement in fast charging capability can be attributed to the reduction in ion transport resistance and the increase in active surface area . In addition, the capacity retention rate of the alternative binder system was 82.6% after 1500 cycles.
Pradhan et al. synthesized a lithium borate-based aqueous polyelectrolyte binder for graphite anode. The lower LUMO energy level of the binder allows it to be preferentially reduced before the electrolyte or lithium salt is degraded, forming a thinner and more conductive borate-rich SEI. The strong borate-rich SEI and Li + -containing binder have low lithiation / desolvation activation energy and high Li + diffusion coefficient in graphite . As shown in Figure 4(c) , the negative electrode half-cell using the new binder has a discharge capacity of 73 mA·h/g at 10C, which is 3 times higher than that of the battery using PVDF and CMC-SBR binder anodes, and can achieve more than 1000 cycles and high capacity retention.
Researchers have also used other methods to optimize fast charging capabilities . For example, Chen et al. used laser perforation to enhance the transport of lithium ions within the porous structure of the electrode, thereby improving the fast charging capability of the graphite negative electrode . Kim et al. enhanced the fast charging capability of the graphite negative electrode by using an amorphous Al 2 O 3 coating on the surface of the active material . In comparison, optimizing the binder system does not require additional lasers and expensive processing steps in the coating process, reducing production costs . In addition, most of the newly developed binder systems are only at the laboratory research stage , and commercial applications still need further verification .
4Binder for silicon negative electrode
Silicon has attracted much attention due to its high theoretical specific capacity , abundant natural reserves and suitable working voltage . However, Si negative electrodes have inherent defects, such as large volume change during charge and discharge, poor electron migration ability, and unstable SEI, which hinder their practical application . To solve the above problems of Si negative electrodes, researchers have proposed various innovative strategies, such as controlling the morphology and size of silicon active materials , surface coating , etc.
Although these strategies have been proven to improve the electrochemical performance of Si anodes, their manufacturing process is complex and costly . Binders, as key components of electrodes, play a vital role in maintaining electrode morphology and mechanical stability . The development of functional binders has become a low-cost strategy that not only adheres active material particles and conductive agents to the current collector, reduces the volume expansion of Si , but also enhances ionic and electronic conductivity .
Traditional binders used for graphite anodes, such as PVDF and SBR, rely on weak van der Waals forces to achieve adhesion and are difficult to maintain the integrity of Si anodes . Therefore, improving the adhesion of binders to Si particles and Cu current collectors is the key to improving the cycle stability of Si anodes. Researchers have developed a variety of new binders such as sodium alginate , PAA/CMC composites, gum arabic, and guar gum. These binders have the strength , flexibility and bonding properties required for Si anode systems , can withstand large volume changes, and improve the stability of Si anodes .
By properly regulating the intermolecular bonds and structures of polymer binders, broken Si particles can be effectively stabilized, thereby ensuring the stability of SEI . For example, Wang et al. innovatively developed a new inorganic binder , lithium metasilicate (LS) . As shown in Figure 5(a) , due to the presence of silicon in the LS skeleton, LS binder has good interfacial compatibility with Si nanoparticles (SiNPs) . The interaction between LS binder and SiNPs can produce a strong adhesion effect and enhance the cycle stability of Si negative electrode . At 0.84A/g , after 100 cycles, the average discharge specific capacity of Si negative electrode using LS binder is 2123mA·h/g.
Lim et al. introduced phenyl isocyanate (MDI) into waterborne polyurethane (WPU ) binder , and the two were cross-linked to form a network structure (TMPU) binder, the structure of which is shown in Figure 5(b) . Thanks to the ionic part and network structure in the polymer chain, TMPU binder not only has solvent resistance, but also has excellent mechanical properties .
Wu et al. prepared a multifunctional aqueous borax-based binder (SBG) . As shown in Figure 5(c) , the three-dimensional network structure of the SBG binder makes the stress distribution uniform, while its self-healing ability promotes stress dissipation and mechanical damage repair, thereby improving the damage tolerance of the electrode . These carefully designed structures give the binder superior mechanical strength and self-healing ability, and can also serve as a strong scaffold to alleviate volume changes and dissipate concentrated mechanical strain .
Although enhancing the mechanical properties of polymer binders is considered to be an effective means to improve the stability of SEI on Si anodes , the mechanism by which the chemical properties of binders affect SEI is still unclear . Jin et al. designed a zwitterionic binder (PTA) that can control the chemical composition and spatial distribution of the SEI layer, and elucidated the mechanism by which zwitterionic binders affect the solvation configuration and stability of the anode - electrolyte interface. Cations and anions coexist at the molecular level, and the charged microenvironment formed by zwitterions changes the solvation environment of the Si anode, weakening the interaction between Li + and the solvent. As shown in Figure 5(d) , in this binder-regulated solvation environment, when the binder / anion participates in the formation of SEI , the reduction of the electrolyte is significantly suppressed, which helps to form a thin and tough SEI on the Si anode .
The first coulombic efficiency (ICE) is a key indicator for evaluating Si anodes . Si anodes usually exhibit an ICE of 70%~85% , which is significantly lower than the ICE of 90%~95% of commercial graphite anodes . Zhu et al. synthesized a polyacrylic acid (LiPAA) binder capable of precise pre-lithiation through a simple and environmentally friendly method, which has strong bonding strength and excellent mechanical strength . The addition of additional lithium sources to LiPAAs can significantly improve the ionic conductivity and contribute to the stability of the SEI layer . Among the LiPAAs studied , LiPAA-3 has excellent mechanical and electrochemical properties. As shown in Figure 5(e) , the specific capacity of the Si-LiPAA-3 electrode is as high as 3553mA·h/g, and the initial coulombic efficiency is 90.1%, which is better than the performance of most micron-sized Si anodes .
Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials,electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email:janice@canrd.com
Phone/Wechat/WhatsApp/Skype:+86 18928276992
Website : www.canrud.com











No comments:
Post a Comment