Introduction to Lithium-ion Batteries (LIB)
Lithium-ion batteries are mainly composed of four parts: positive electrode materials, negative electrode materials, separators, and electrolytes. The positive electrode materials must participate in chemical reactions and also provide Li+ as a lithium ion source; the negative electrode materials are also an important component of the battery, generally carbon, lithium titanate, and silicon-based alloy materials; the main function of the separator is to prevent the positive and negative electrode materials from directly contacting each other and causing a short circuit, and it is generally a porous membrane material such as polyethylene or polypropylene; the role of the electrolyte is to provide a channel for the transmission of lithium ions and promote the reversible reaction of the electrode. It is mainly composed of electrolyte lithium salts, non-aqueous organic solvents, and necessary additives.
Figure 1. Applications of lithium-ion batteries
The charging and discharging principle diagram of lithium-ion batteries is shown in Figure 2. Simply put, lithium-ion batteries use internal ion deintercalation and chemical reactions to transfer charges, thereby achieving the mutual conversion of chemical energy and electrical energy. During charging, Li+ is separated from the positive electrode under the action of the electric field, and the Li+ in the electrolyte moves through the diaphragm to the negative electrode and embeds into the negative electrode. At the same time, in order to maintain electrical neutrality, a large number of electrons will gather in the external circuit, realizing the conversion of electrical energy into chemical energy, and the discharge process is the opposite.
Figure 2. Working mechanism of lithium-ion battery
Cathode materials of lithium-ion batteries
Positive electrode materials play a decisive role in the energy density and cycle life of batteries, and are therefore of vital importance. Ideal positive electrode materials should meet the following conditions: (1) high operating voltage and higher discharge specific capacity; (2) good high-rate performance of charge and discharge, higher electronic conductivity and ionic conductivity, and rapid charge and discharge; (3) high stability and long cycle life, the material maintains structural stability and thermal stability during long-term electrochemical reactions and does not react with the electrolyte; (4) good safety, low heat released during charge and discharge; (5) easy preparation and storage, the relevant synthesis process is production-feasible, and the storage conditions are simple and easy to implement; (6) low raw material cost, abundant resources, and environmentally friendly.
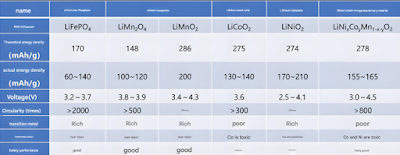
Positive electrode materials are usually composed of three parts: positive electrode active material, conductive agent and organic binder. According to the different components of positive electrode active material, common positive electrode materials can be divided into five categories, namely lithium iron phosphate (LiFePO 4 ), lithium manganese oxide (LiMn 2 O 4 and LiMnO 2 ), lithium cobalt oxide (LiCoO 2 ), lithium nickel oxide (LiNiO 2 ), and nickel cobalt manganese oxide ternary material (LiNi x Co y Mn 1-xy O 2 ). Table 1 shows the performance comparison of these positive electrode materials. It can be seen from the table that ternary positive electrode materials have relatively excellent performance parameters, so they are gradually widely used.
Table 1 Comparison of cathode material performance
Ternary cathode materials (NCM)
Ternary materials refer to nickel-cobalt-manganese ternary positive electrode materials (LiNi x Co y Mn z O 2 , where x+y+z=1), that is, a new material obtained by replacing part of Co with Ni and Mn on the basis of LiCoO 2. It inherits the stability of LiCoO 2 , the high reversible capacity of LiNiO 2 , and the high safety of LiMnO 2. Compared with lithium cobalt oxide, the content of Co in ternary materials is reduced, which reduces the cost. These advantages make ternary materials one of the new lithium-ion battery positive electrode materials with the broadest development prospects.
The electrochemical properties of ternary materials vary greatly depending on the content of nickel, cobalt and manganese. According to the different contents of transition metal elements, the common ternary materials can be divided into two categories: the first category is Ni:Mn isomeric type, namely LiNi 0.4 Co 0.2 Mn 0.4 O 2 (NCM 424) and LiNi 1/3 Co 1/3 Mn 1/3 O 2 (NCM 111), and the other category is high nickel type, namely LiNi 0.5 Co 0.2 Mn 0.3 O 2 (NCM 523), LiNi 0.6 Co 0.2 Mn 0.2 O 2 (NCM 622), LiNi 0.7 Co 0.2 Mn 0.1 O 2 (NCM 721) and LiNi 0.8 Co 0.1 Mn 0.1 O 2 (NCM 811).
Among them, Ni is mainly an electrochemical change element. The higher the proportion of Ni, the higher the capacitance of the material; however, when the Ni content increases to a certain extent, the structural stability and capacity retention rate of the material will decrease. Co improves electrochemical polarization and increases the coulombic efficiency of the battery, but too much Co will increase the material cost. Mn can enhance the stability and safety of the material while reducing the price cost.
The structure of ternary cathode materials
The ternary cathode material has an a-NaFeO2-type layered rock salt structure similar to that of the LiCoO2 material , and belongs to the hexagonal R-3m space group. Figure 3 shows its crystal structure. The stacking mode of oxygen anions is cubic close packing, while lithium ions and transition metal ions alternately occupy the octahedral gaps according to the stoichiometric ratio, and the distribution of each ion is layered. Among them, Li is in the 3a position in the crystal structure, O is in the 6c position, and Ni, Co, and Mn are in the 3b position, together forming the MO6 octahedron. Because the radii of different transition metal ions are different, the crystal parameters of the ternary cathode material system change with different x values. As the Ni content in the material continues to increase, the Co content gradually decreases, and the lattice constants a and c gradually increase.
In the ternary material, Ni and Co participate in the electrochemical reaction, among which Ni mainly exists in the valence state of Ni 2+ /Ni 4+ . Its redox process corresponds to the extraction and embedding of Li+, which can effectively increase the number of embeddable Li + in the NCM material and improve the discharge capacity of the material; Co mainly exists in the form of Co 3+ /Co 4+ , which affects the ionic conductivity of the material, that is, the extraction and embedding rate of Li between layers; Mn serves as a skeleton to stabilize the structure of the material lattice, and mainly exists as Mn 4+ .
Figure 3. Schematic diagram of the structure of LiNi x Co y Mn 1-xy O 2
Research progress of ternary cathode materials
Although ternary cathode materials have many advantages, they also have some inevitable intrinsic defects. For example, the cycle structure changes under high voltage, resulting in poor cycle performance, the mixed cation arrangement and low electronic conductivity lead to poor rate performance, oxygen vacancies and easy structural collapse during charge and discharge, etc.
In response to the problems of ternary cathode materials, researchers have adopted a variety of solutions. First, improve the preparation method. There are many methods for preparing ternary cathode materials, and the size, micromorphology, and electrochemical properties of materials prepared by different methods are different. Secondly, the electrochemical properties of the materials are improved through modification measures, mainly through ion doping, surface coating, structural design and other methods.
Ion doping refers to a modification method that introduces foreign ions into the material structure to improve the structural stability of the material. Generally speaking, Al, Ti, Zr, Mg, B, F, W, Mo, Ga, Nb, Ca, etc., these elements can be doped alone or in combination. Generally speaking, element doping techniques include cation doping, anion doping, cation co-doping, and anion-cation co-doping. Different doping ions play different roles in the material structure. A large number of studies have shown that the proper introduction of doping elements can stabilize the structure, reduce cation mixing, expand the interlayer spacing, and inhibit harmful phase changes.
It has been reported in the literature that after doping B 3+ in NCM , the morphology inside the spherical particles changes. As shown in Figure 4, the primary particles are columnar along the radial direction, which is different from the equiaxed shape in the undoped material. This change in grain morphology has a great influence on the performance of the material during the charge and discharge cycle. After 100 cycles, there is no radial intergranular crack in the matrix of the material doped with B 3+ , while multiple radial cracks appear in the undoped material.
Figure 4. Image of B-doped high-nickel NCM material changing the grain morphology of primary particles
Surface coating refers to adding a covering layer on the surface of the material. This modification method has a great influence on the interface performance of the ternary cathode material, which can prevent the active material from directly contacting the electrolyte and avoid decomposition or oxidation of the electrolyte. At the same time, the surface coating can inhibit the damage of mechanical stress and avoid the generation of cracks and particle breakage during the cycle. Commonly used coating materials include oxides (Al 2 O 3 , ZrO 2 , TiO 2 , B 2 O 3 , MoO 3 , WO 3 , CeO 2 ), phosphates (aluminum phosphate, iron phosphate, nickel phosphate, etc.), fluorides (aluminum fluoride, calcium fluoride, etc.), electronic conductor materials (mainly carbon nanotubes, graphene and some organic matter), ion conductor materials (Li 7 La 3 Zr 2 O 12 , Li 0.33 La 0.557 TiO 3 , Li 1.4 Al 0.4 Ti 1.6 (PO 4 ) 3 , etc.), and conductor materials (LiFePO 4 , LiMn 2 O 4, etc.).
It has been reported in the literature that after the ternary material is coated with reduced graphene and carbon nanotubes, a 2D and 3D electronic conductive network is formed on the surface of the particles, as shown in Figure 5. Without affecting the migration of Li + , the positive electrode electronic conductivity is greatly improved.
Figure 5. SEM and TEM images of graphene and carbon nanotube-coated NCM
Designing the components of materials to obtain cathode materials with specific components can also improve the electrochemical performance of the materials. Since high-nickel ternary materials have the advantage of high capacity, and low-nickel ternary materials have good stability, through a certain process, low-nickel materials can be coated on the surface of high-nickel materials, and a cathode material with both advantages may be synthesized.
Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials, electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email: contact@canrd.com Phone/Wechat/WhatsApp: +86 19867737979
Canrd Official Web Canrd Company Vedio Canrd Company profile
Website : www.canrud.com














No comments:
Post a Comment