1.Basics of Pole Design
The lithium battery electrode is a coating composed of particles, which is evenly coated on the metal current collector. The lithium-ion battery electrode coating can be regarded as a composite material, which mainly consists of three parts:
(1) Active substance particles;
(2) A phase in which the conductive agent and the binder are mixed (carbon glue phase);
(3) Pores, filled with electrolyte.
The volume relationship of each phase is expressed as:
Porosity + active matter volume fraction + carbon gel phase volume fraction = 1
The design of lithium battery pole pieces is very important. Here we briefly introduce the basic knowledge of lithium battery pole piece design.
(1)Theoretical capacity of electrode materials
The theoretical capacity of the electrode material, that is, the capacity that can be provided assuming that all lithium ions in the material participate in the electrochemical reaction, is calculated by the following formula:
This calculated value is only the theoretical gram capacity. To ensure the reversibility of the material structure, the actual lithium ion deintercalation coefficient is less than 1. The actual gram capacity of the material is:
Actual gram capacity of material = lithium ion deintercalation coefficient × theoretical capacity
(2) Battery design capacity and electrode surface density
The battery design capacity can be calculated by the following formula: Battery design capacity = coating surface density × active material ratio × active material gram capacity × pole piece coating area
Among them, the surface density of the coating is a key design parameter. When the compaction density remains unchanged, the increase in the surface density of the coating means that the thickness of the electrode increases, the electron transmission distance increases, and the electronic resistance increases, but the increase is limited. In thick electrodes, the increase in the migration impedance of lithium ions in the electrolyte is the main reason affecting the rate characteristics. Considering the porosity and the tortuosity of the pores, the migration distance of ions in the pores is many times greater than the thickness of the electrode.
(3)Negative electrode-positive electrode capacity ratio N/P
The ratio of the negative electrode capacity to the positive electrode capacity is defined as:
N/P should be greater than 1.0, generally 1.04~1.20, which is mainly for safety design to prevent the negative electrode side lithium ions from precipitating due to lack of receiving source. The process capability, such as coating deviation, should be considered during design. However, when N/P is too large, the irreversible capacity of the battery is lost, resulting in low battery capacity and reduced battery energy density.
For lithium titanate negative electrode, the positive electrode excess design is adopted, and the battery capacity is determined by the capacity of the lithium titanate negative electrode. The positive electrode excess design is conducive to improving the high temperature performance of the battery: the high temperature gas mainly comes from the negative electrode. When the positive electrode excess design is used, the negative electrode potential is lower, and it is easier to form a SEI film on the surface of lithium titanate.
(4)Compacted density and porosity of coating
During the production process, the coating compaction density of the battery electrode is calculated by the following formula:
Taking into account the extension of the metal foil when the pole piece is rolled, the surface density of the coating after rolling is calculated by the following formula.
As mentioned above, the coating consists of an active material phase, a carbon gel phase and pores, and the porosity can be calculated by the following formula.
Among them, the average density of the coating is:
Lithium battery electrode is a coating composed of powder particles. Since the powder particles have a rough surface and irregular shape, there must be pores between the particles when they are stacked, and some particles themselves have cracks and pores. Therefore, the volume of the powder includes the volume of the powder itself, the pores between the powder particles, and the pores inside the particles. Therefore, there are multiple corresponding methods for expressing the density and porosity of the electrode coating.
The density of powder particles refers to the mass of powder per unit volume. According to the volume of powder, it is divided into three types: true density, particle density, and bulk density. The definitions of various densities are as follows:
a.True density refers to the density obtained by dividing the mass of the powder by the volume excluding the voids inside and outside the particles (real volume). That is, the density of the substance itself is obtained after excluding the volume occupied by all voids.
b.Particle density refers to the density obtained by dividing the mass of the powder by the volume of the particle including open pores and closed pores. That is, the density of the particle itself is obtained by excluding the gaps between the particles, but not excluding the tiny pores inside the particles.
c.Bulk density, or coating density, is the density obtained by dividing the mass of the powder by the volume of the coating composed of the powder. The volume used includes the total volume of the pores of the particles themselves and the gaps between the particles.
For the same powder, true density > particle density > bulk density.
The porosity of powder is the ratio of pores in the powder particle coating, that is, the ratio of the volume occupied by the gaps between powder particles and the pores of the particles themselves to the total volume of the coating, and is usually expressed as a percentage. The porosity of powder is a comprehensive property related to factors such as particle morphology, surface state, particle size and particle size distribution. The size of its porosity directly affects the infiltration of electrolyte and lithium ion transmission. Generally speaking, the larger the porosity, the easier the electrolyte infiltration and the faster the lithium ion transmission. Therefore, in the design of lithium batteries, it is sometimes necessary to measure the porosity, which is usually measured by mercury injection method, gas adsorption method, etc. It can also be obtained by density calculation. When different densities are used for calculation, the meaning of porosity is also different.
When the porosity is calculated based on the true density of active materials, conductive agents, and binders, the calculated porosity includes the voids between particles and the voids inside particles. When the porosity is calculated based on the particle density of active materials, conductive agents, and binders, the calculated porosity includes the voids between particles, but not the voids inside particles. Therefore, the pore size of lithium battery pole pieces is also multi-scale. Generally, the voids between particles are in the micron size, while the voids inside particles are in the nanometer to submicron size.
In porous electrodes, the relationship between transport properties such as effective diffusivity and conductivity can be expressed as
follows:
Among them, D0 represents the intrinsic diffusion (conductivity) of the material itself, ε is the volume fraction of the corresponding phase, and τ is the tortuosity of the corresponding phase. In the macroscopic homogeneous model, the Bruggeman relationship is generally used, and the coefficient ɑ=1.5 is taken to estimate the effective physical properties of the porous electrode.
The electrolyte is filled in the pores of the porous electrode, and lithium ions are conducted through the electrolyte in the pores. The conductivity of lithium ions is closely related to the porosity. The greater the porosity, the higher the volume fraction of the electrolyte phase, and the greater the effective conductivity of lithium ions. In the positive electrode, electrons are transmitted through the carbon gel phase, and the volume fraction and tortuosity of the carbon gel phase directly determine the effective conductivity of electrons.
Porosity and the volume fraction of the carbon colloid phase are contradictory. A large porosity will inevitably lead to a decrease in the volume fraction of the carbon colloid phase. Therefore, the effective conduction characteristics of lithium ions and electrons are also contradictory, as shown in Figure 2. As the porosity decreases, the effective conductivity of lithium ions decreases, while the effective conductivity of electrons increases. In electrode design, how to balance the two is also critical.
Figure 2 Schematic diagram of the relationship between porosity and lithium ion and electron conductivity
2.Types and detection of pole piece defects
At present, in the process of battery electrode preparation, more and more online detection technologies are being used to effectively identify product manufacturing defects, eliminate defective products, and provide timely feedback to the production line, automatically or manually adjust the production process, and reduce the defective rate.
Commonly used online detection technologies in pole piece manufacturing include slurry property detection, pole piece quality detection, size detection and other aspects, such as: (1) The online viscometer is directly installed in the coating storage tank to detect the rheological properties of the slurry in real time and detect the stability of the slurry; (2) X-rays or β-rays are used to directly measure the surface density of the coating during the coating process. The measurement accuracy is high, but the radiation is large, the equipment price is high and the maintenance is difficult; (3) Laser online thickness measurement technology is used to measure the thickness of the pole piece. The measurement accuracy can reach ±1.0μm, and it can also display the measured thickness and thickness change trend in real time, which is convenient for data tracing and analysis; (4) CCD visual technology is used to detect the surface defects of the pole piece, that is, a linear array CCD is used to scan the object to be measured, and the image is processed and the defect category is analyzed in real time to achieve non-destructive online detection of pole piece surface defects.
In-line inspection technology is a quality control tool, and it is also essential to understand the correlation between defects and battery performance in order to determine the pass/fail criteria for semi-finished products.
The following section briefly introduces a new method for detecting surface defects of lithium-ion battery electrodes - infrared thermal imaging technology, as well as the relationship between these different defects and electrochemical performance. Reference is made to the in-depth research conducted by D. Mohanty et al.
(1) Common defects on the electrode surface
Figure 3 shows the common defects on the surface of lithium-ion battery electrodes. The left side is an optical image and the right side is an image captured by a thermal imager.
Figure 3 Common defects on the electrode surface: (a, b) bulges/agglomerates; (c, d) material dropouts/pinholes; (e, f) metallic foreign matter; (g, h) uneven coating
(a, b) Protrusions/agglomerates, which can occur if the slurry is not stirred evenly or the coating feed rate is unstable. Agglomerates of binder and carbon black conductive agent result in low active ingredient content and light pole piece weight.
(c, d) Dropouts/pinholes, these defective areas without coating, are usually generated by bubbles in the slurry. They reduce the amount of active material and expose the current collector to the electrolyte, thereby reducing the electrochemical capacity.
(e, f) Metal foreign matter, metal foreign matter introduced into the slurry, equipment, or environment, is extremely harmful to lithium batteries. Larger metal particles directly pierce the diaphragm, causing a short circuit between the positive and negative electrodes. This is a physical short circuit. In addition, when metal foreign matter is mixed into the positive electrode, the positive electrode potential increases after charging, the metal dissolves, diffuses through the electrolyte, and then precipitates on the surface of the negative electrode, eventually piercing the diaphragm to form a short circuit. This is a chemical dissolution short circuit. The most common metal foreign matter on battery factory sites are Fe, Cu, Zn, Al, Sn, SUS, etc.
(g, h) Uneven coating, such as insufficient slurry stirring and the appearance of streaks when the particle size is large, resulting in uneven coating, which will affect the consistency of battery capacity and even cause streaks without coating at all, affecting both capacity and safety.
(2)Pole surface defect detection technology Infrared (IR) thermal imaging technology is used to detect tiny defects on dry electrodes that may damage the performance of lithium-ion batteries. During online inspection, if electrode defects or contaminants are detected, they are marked on the electrode, removed in subsequent processes, and fed back to the production line to adjust the process in time to eliminate defects. Infrared is an electromagnetic wave with the same nature as radio waves and visible light. The technology of using a special electronic device to convert the temperature distribution on the surface of an object into an image visible to the human eye and display the temperature distribution on the surface of the object in different colors is called infrared thermal imaging technology. This electronic device is called an infrared thermal imager. All objects above absolute zero (-273°C) emit infrared radiation.
As shown in Figure 4, the infrared thermal imager (IR Camera) uses an infrared detector and an optical imaging lens to receive the infrared radiation energy distribution pattern of the target object to be measured and reflects it on the photosensitive element of the infrared detector, thereby obtaining an infrared thermal image. This thermal image corresponds to the heat distribution field on the surface of the object. When there are defects on the surface of an object, the temperature in this area will shift. Therefore, this technology can also be used to detect defects on the surface of an object, especially for some defects that cannot be distinguished by optical detection methods. During the online detection of lithium-ion battery dry pole pieces, the pole piece is first irradiated by a flash lamp, and the surface temperature changes. Then the surface temperature is detected by a thermal imager. The thermal distribution image is visualized, and the image is processed and analyzed in real time. When surface defects are detected, they are marked in time. D. Mohanty's research installed the thermal imager at the exit of the coating machine drying oven to detect the temperature distribution image on the pole piece surface.
Figure 4 Schematic diagram of the thermal imager detecting the surface of the pole piece
Figure 5 (a) is a temperature distribution diagram of the coating surface of the NMC positive electrode detected by a thermal imager, which contains a very small defect that cannot be distinguished by the naked eye. The temperature distribution curve corresponding to the line segment is shown in the inset, and a temperature spike appears at the defect point. The box corresponding to the image in Figure 5 (b) shows a local temperature increase, which corresponds to the defect on the surface of the electrode. Figure 6 is a temperature distribution diagram of the surface of the negative electrode, showing the existence of defects, where the peak of temperature increase corresponds to bubbles or agglomerates, and the area of temperature decrease corresponds to pinholes or material dropouts
Figure 5 Thermal imaging temperature distribution of the positive electrode surface
Figure 6 Thermal imaging temperature distribution of the negative electrode surface
It can be seen that thermal imaging detection of temperature distribution is a good means of detecting surface defects of pole pieces and can be used for quality control of pole piece manufacturing.
3.Impact of electrode surface defects on battery performance
(1)Impact on battery rate capacity and coulombic efficiency
Figure 7 shows the effect of aggregates and pinholes on battery rate capacity and coulombic efficiency. Agglomerates can actually increase battery capacity, but reduce coulombic efficiency. Pinholes reduce battery capacity and coulombic efficiency, and the coulombic efficiency decreases significantly at high rates.
Figure 7 Effects of cathode aggregates and pinholes on battery rate capacity and coulombic efficiency
Figure 8 shows the effect of uneven coating and metal foreign matter Co and Al on battery rate capacity and coulomb efficiency. Uneven coating reduces battery unit mass capacity by 10%-20%, but the overall battery capacity drops by 60%, indicating that the mass of active matter in the electrode has been significantly reduced. Metal Co foreign matter reduces capacity and coulomb efficiency, and even at high rates of 2C and 5C, there is no capacity at all. This may be due to the formation of alloys by metal Co in the electrochemical reaction, which hinders lithium removal and lithium insertion, or it may be due to metal particles blocking the pores of the diaphragm, causing micro-short circuits.
Figure 8 Effects of uneven positive electrode coating and metallic foreign matter Co and Al on battery rate capacity and coulombic efficiency
Summary of positive electrode defects:Agglomerates in the cathode electrode coating reduce the Coulombic efficiency of the battery.Pinholes in the cathode coating reduce the Coulombic efficiency, leading to poor rate performance, especially at high current densities.Non-uniform coatings show poor rate performance.Metal particle contamination can cause micro short circuits and therefore can significantly reduce battery capacity.
Figure 9 shows the effect of negative electrode leakage stripes on battery rate capacity and coulombic efficiency. When the negative electrode has leakage stripes, the battery capacity is significantly reduced, but the gram capacity is not significantly reduced, and the effect on coulombic efficiency is not significant.
Figure 9 Effect of negative electrode leakage stripes on battery rate capacity and coulombic efficiency
(2)Impact on battery rate cycling performance
Figure 10 shows the effect of the electrode surface defects on the battery rate cycle, and the effect results are summarized as follows:
Aggregates: At 2C, the capacity retention rate of the battery without defective electrode sheet after 200 cycles is 70%, while that of the defective battery is 12%. At 5C cycle, the capacity retention rate of the battery without defective electrode sheet after 200 cycles is 50%, while that of the defective battery is 14%.
Pinhole: The capacity decay is obvious, but not as fast as the decay of agglomerate defects. The capacity maintenance rates of 2C and 5C after 200 cycles are 47% and 40% respectively.
Metal foreign matter: The capacity of metal Co foreign matter is almost 0 after several cycles, and the capacity of metal foreign matter Al foil decays significantly after 5C cycle.
Leakage foil stripes : Under the same leakage foil area, compared with one large stripe (capacity retention rate of 47% after 200 cycles at 5C), the battery capacity decays faster with multiple small stripes (capacity retention rate of 7% after 200 cycles at 5C). This shows that the more stripes there are, the greater the impact on battery cycling.
Key issues in super-fast charging of lithium-ion power batteries
In recent years, in order to limit the impact of climate change and air pollution, the widespread use of lithium-ion batteries in pure electric vehicles is accelerating. However, compared with traditional fuel vehicles, problems such as range anxiety and long charging time have become the main problems hindering the development of electric vehicles. Therefore, the improvement of fast charging capability has become a common development goal for battery manufacturers and vehicle manufacturers. However, studies have shown that low temperature and high-rate charging will cause the battery's capacity and output power to decay faster; on the other hand, the large amount of heat generated by the battery during charging is difficult to dissipate evenly and effectively, which will also cause accelerated decay and other safety issues. Figure 1 shows the factors that affect the fast charging of lithium-ion batteries from the atomic level to the vehicle system level. This article focuses on the review and summary of existing literature and analyzes the key technical limiting factors at each level.
Figure 1 Factors affecting fast charging of lithium-ion batteries at different levels
Electric vehicle charging is classified into AC and DC, of which DC charging is faster. Tesla was the first company to use 120kW fast charging; Bosch released a 350kW fast charging plan in 2017, which was implemented in the "Taycan" in 2019. Since the voltage of the current automotive battery pack is around 400V, the 350kW high-power charging requires a higher voltage for the pack to avoid problems such as excessive current and excessive heat generation. Bosch's "Taycan" and Audi's e-tron GT concept car (charging power of 350kW) are both equipped with 800V lithium-ion battery packs. In December 2018, a joint research team of BMW, Bosch and Siemens achieved 450kW CCS mode fast charging on two test vehicles in Germany.
Although great progress has been made in the research of improving the charging power of electric vehicles, these fast charging technologies are not suitable in all cases. Depending on the specific operating conditions and charging environment of the electric vehicle, the charging power will gradually decay during the continuous charging process. In addition, in the fast charging mode, due to safety and other factors, the battery can usually only be charged to 80% of the power; at higher power, the charging rate will gradually decrease to avoid overcharging. In addition, the charging power is also limited by the battery management system (BMS). The industry is increasingly interested in the field of battery fast charging, and it is necessary to understand the rate-determining steps of different charging methods and their impact on battery life. This article aims to establish the connection between microscopic processes, material properties, battery and pack design, and charging strategy optimization based on the multi-scale and multi-disciplinary characteristics of fast charging.
The principle of fast battery charging
An ideal battery should exhibit long life, high energy density, and high power density, so that it can be quickly charged and recharged at any location and at any temperature to meet the requirements of long-distance driving of electric vehicles. However, there is a trade-off relationship between these physical properties, and the influence of the temperature of materials and equipment determines the use threshold of the battery. When the temperature drops, both the charging rate and the maximum voltage should be reduced to ensure safety, which makes temperature a key limiting factor for fast charging. Among them, the risk of lithium plating increases significantly as the temperature decreases. Although many researchers have pointed out that lithium plating often occurs at temperatures below 25°C, it is also prone to occur at high temperatures, especially at high charging rates and high energy densities. In addition, the relationship between fast charging efficiency and temperature is also very close. The charging efficiency of a 50kW charging pile at 25°C is 93%, but the charging efficiency at -25°C is as low as 39%, mainly because the BMS limits the rated power at low temperatures.
Common lithium-ion batteries are mainly composed of graphite negative electrode, lithium metal oxide positive electrode, electrolyte, current collector, and porous separator. As shown in Figure 2, during charging, Li+ is transferred from the positive electrode to the negative electrode through the electrolyte. The main transmission paths are: 1) through the solid electrode; 2) through the electrode/electrolyte interface of the positive and negative electrodes; 3) through the electrolyte, including the solvation and desolvation of Li+. However, improper battery use conditions often cause a series of side reactions that affect performance and life. In addition, the charge and discharge rate, battery internal resistance, and battery polarization will affect the thermal characteristics of the battery, such as increasing heat generation, reducing charging efficiency and safety, etc.
Figure 2 Schematic diagram of lithium ion transmission a) charging, b) discharging
A large number of studies have shown that the attenuation of the positive electrode and the growth of the positive electrode CEI film have no effect on the fast charging speed of traditional lithium-ion systems, so the negative electrode becomes the main focus during the charging process. Under certain circumstances, lithium metal may continue to precipitate into lithium dendrites, and even pierce the separator to cause internal short circuits. Factors affecting lithium deposition and deposition structure include the diffusion rate of lithium ions in the negative electrode, the electrolyte concentration gradient at the negative electrode interface, the metal salt deposition of the current collector, and the side reactions at the electrode/electrolyte interface. Studies have shown that the performance of the negative electrode during lithium deposition can be attributed to the effect of the current at the beginning of lithium deposition on the negative electrode surface density internal resistance. Reducing the negative electrode internal resistance through battery design is very important for improving the fast charging capability of the battery. In addition, the temperature effect is also very important. Too low or too high temperature is considered to be unfavorable to the battery, but a higher battery temperature during fast charging is beneficial to its own balance, especially for high specific energy batteries. The effect of electrode thickness on charging performance also needs to be paid attention to. Thin electrodes are often considered to be ideal for lithium ion transmission. When the electrode is thickened, it becomes important to ensure sufficient lithium ion concentration at the electrode/electrolyte interface to maintain overpotential stability and reduce the possibility of lithium deposition. During fast charging of thick electrode batteries, lithium salts may be deposited at the current collector, resulting in unbalanced electrode utilization and an increase in the current density of the separator negative electrode.
Attenuation Effect
1) Temperature influence
The heat generation of lithium-ion batteries can be divided into reversible and irreversible processes. The expression of irreversible heat generation Qirr is as follows:
U is the open circuit voltage, Vbat is the battery voltage, and I is the current (when charging). Most of the irreversible heat comes from the internal resistance heat generation:
Where R is the internal resistance of the battery. Joule heat is proportional to the square of the current, so when fast charging, the current increases and the irreversible heat increases significantly. The reversible heat Qrev comes from the entropy change in the electrochemical reaction, also known as entropy heat, and its expression is:
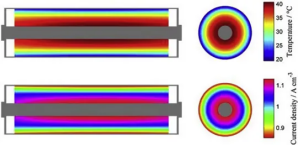
In lithium-ion batteries, the heat distribution and dissipation of soft-pack, cylindrical, and square-shell batteries are uneven: for example, the surface thermal conductivity of some battery materials is poor, so their heat will accumulate more in the core relative to the surface. In addition, the current density and heat generation rate are also different at different positions of the battery. These inconsistencies are further amplified in large-size batteries. As shown in Figure 3, the temperature at the center of the cylindrical battery is significantly higher than the surface. For soft-pack or square batteries, as shown in Figures 4 and 5, the temperature at the tab is significantly higher than other locations. In addition, because the positive aluminum current collector has a greater resistance than the negative copper current collector, the temperature of the positive tab is often higher than that of the negative tab.
Figure 3 Simulation results of temperature and current density distribution inside cylindrical battery
Figure 4 Surface temperature changes of soft-pack batteries during 5C constant current discharge: a) simulation results and b) measurement results at t=250s; c) simulation results and d) measurement results at t=667s; e) 3D distribution of internal temperature
Figure 5 Temperature distribution of LFP battery at a) 1C, b) 2C and c) 5C discharge
The uneven distribution of heat generation not only exists in the battery cells, but also requires more attention to the design of the thermal management system at the battery pack level, because it has a significant impact on the temperature distribution within the pack. Over time, the different aging paths of battery cells will also have a great impact on the uniformity of heat generation in the pack, because the increase in internal resistance of different batteries is different. To solve this problem, the design of the thermal management system will be introduced in Part 6.
Many aging mechanisms in lithium-ion batteries are temperature-dependent. At high temperatures, the SEI film accelerates growth at the anode, becoming more porous and unstable. At low temperatures, ion diffusion and reaction rates slow down, increasing the likelihood of lithium precipitation and dendrite growth. Almost all aging reactions are accelerated at high temperatures; low temperatures can reduce the rate of side reactions but also reduce the diffusion of active substances, which can accelerate decay if lithium metal precipitates. In addition, increased polarization at low temperatures can lead to increased heat generation and reduced energy efficiency. Under most operating conditions, the growth of the SEI film at the anode/electrolyte interface is the main decay mechanism. The SEI film increases the internal resistance of the battery, reduces power, and leads to capacity decay. At high temperatures (60°C or higher), SEI components dissolve and decompose, destroying the integrity of the anode protective film. In extreme cases, when the battery temperature exceeds the safety threshold, thermal runaway may occur.
2)Impact of lithium precipitation
Lithium deposition refers to the Faraday side reaction in which lithium ions in the electrolyte are deposited as lithium metal on the negative electrode, rather than being embedded in the negative electrode particles. Lithium deposition may occur when the negative electrode potential drops below Li/Li+. During the lithium deposition process, lithium metal will first form a droplet shape to reduce the surface energy, and the surface metal and electrolyte will react rapidly to form a SEI film. As more lithium is deposited under the SEI film until the SEI film ruptures, a new SEI film is formed on the lithium surface, the lithium salt concentration gradually decreases, and the lithium metal begins to grow vertically to the surface of the electrode to form lithium dendrites. Lithium dendrite growth is considered to be one of the worst side reactions. If the dendrite pierces the diaphragm and reaches the positive electrode, the internal short circuit will cause the battery to generate heat rapidly. Lithium metal is more active than the negative electrode, which further brings about internal side reactions, leading to problems such as SEI growth, gas production and electrolyte dissolution.
Researchers have proposed some models for observing lithium precipitation. These include the lithium precipitation model based on the P2D model by Fuller, Doyle and Newman, and the reversible lithium reinsertion process proposed by Arora, Doyle and White. On this basis, Perkins proposed a control-oriented reduced-order model; Hein and Latz proposed a three-dimensional microstructure analytical model. Ren also considered the reversible reinsertion of lithium and the reaction of irreversible lithium (dead lithium) during the battery charging process.
Non-destructive lithium deposition observation technology is very important for practical battery applications. Generally, the tests that can be used for lithium deposition characterization include SEM, TEM, NMR and XRD, but these methods require the battery to be destroyed or a special battery configuration to be used. Commonly used non-destructive lithium deposition observations utilize the external characteristics of the battery, including aging rate, voltage platform of lithium reinsertion, model prediction and other methods. As shown in Figure 6, lithium deposition detection methods based on aging characteristics include (a) Arrhenius equation, (b) capacity and impedance change analysis during the attenuation process, (c) nonlinear frequency domain response analysis and (d) Coulomb efficiency analysis.
Figure 6 Lithium deposition detection method based on aging characteristics
Some of the lithium analyzed will be re-embedded into the negative electrode or dissolved during the discharge process. The relaxation process after the end of charging or the immediate discharge process will produce a new voltage platform, as shown in Figure 7. Voltage differential (DVA) and capacity differential (ICA) are helpful in finding the voltage platform, but these methods require a small discharge rate. Large current will increase polarization and cover the lithium precipitation signal on the voltage curve. The process of lithium precipitation and re-embedding may also cause abnormal heat generation peaks as one of the signals of lithium precipitation.
The increase in battery thickness may also lead to lithium deposition, but the relevant mechanism needs further study. The prediction of lithium deposition using electrochemical models usually depends on the charging conditions. However, these models are too complex and require a lot of calculations, and they need to be further simplified to achieve online detection. A few methods can achieve quantitative in-situ lithium deposition detection after abnormal battery charging. The author believes that the detection method based on abnormal voltage platform is the most promising for application, but there are still high knowledge and technical barriers to its actual application.
Figure 7 Lithium plating detection based on lithium reinsertion.
a) Simulation of overpotential changes during CC-CV charging and standing. Stage I, no lithium deposition on the negative electrode particles; Stage II, lithium deposition begins to occur; Stage III, part of the reversible lithium is re-embedded into the negative electrode or dissolved, and the rest becomes dead lithium; Stage IV, equilibrium state, dead lithium no longer participates in subsequent cycles; b) Voltage differential analysis (DVA); c) Differential capacity analysis (ICA)
3) Mechanical influence
Mechanical pulverization is another important aging phenomenon caused by fast charging, and has been confirmed in a variety of electrode materials (graphite, NMC, LCO, NCA, Si, etc.). Mechanical attenuation can be divided into the following parts according to the scale: rupture inside the electrode particles, separation of electrode particles from conductive carbon and adhesives, separation of active materials from current collectors, and electrode stratification. The main reason for these phenomena is that the gradient distribution of lithium concentration during fast charging causes stress mismatch between components. When the energy release rate or stress exceeds a certain value, cracks will appear in the particles, accompanied by the rupture of the SEI/CEI film. When the strains between the primary particles caused by fast charging cannot match each other, the electrode particles or the particles and the conductive carbon and adhesive will lose contact. The strain mismatch between the electrode material and the current collector can also cause the active material to fall off. High rates can cause serious uneven current density distribution between electrode plates. If there is no external pressure, stratification may occur between the electrode plates.
The effects of mechanical decay on battery performance can be divided into loss of active materials (LAM), loss of active lithium (LLI), and increased impedance. First, cracks can lead to poor electrical contact; second, cracks will expose more fresh surfaces to react with the electrolyte, and the high temperature caused by fast charging will accelerate the above side reactions. These reactions in turn accelerate the growth of SEI, exacerbating impedance increase, LAM and LLI. Finally, the consumption of electrolyte will reduce the wettability of the electrode surface and hinder ion transport. The related positive feedback mechanism can be described as follows: high-rate current leads to crack formation; cracks exacerbate the difference in electron and ion transmission rates, because ions can be transmitted to the cracks through the electrolyte but electrons cannot, which leads to uneven charge state and further exacerbates crack generation. In addition, the author also briefly introduced the research on the effect of particle size on mechanical decay during fast charging, the effect of high rate on secondary particle fracture, and the optimization of fast charging strategy based on mechanical decay limitations.
In general, there are still many issues to be studied about mechanical degradation under fast charging conditions. Different experiments on this issue have produced different conclusions, and there are still controversial views on some important issues, such as the relationship between charging rate and crack generation rate. Mechanical degradation is also generally difficult to decouple from other aging mechanisms. Compared with aging mechanisms such as SEI growth or lithium precipitation, few models have studied the mechanical effects under high currents, and very few of these models have been experimentally verified. The determination of model parameters and boundary conditions has become a major problem hindering the development of mechanical models.
4. Multi-scale fast charging performance design
Fast-charge-induced aging and aging modes are affected by multiple factors, including battery material composition (intrinsic properties of electrode materials and electrolytes), operating conditions (high-rate charge and discharge, extreme voltage and temperature), battery production process, and Pack design. Multi-scale design and composite methods will help develop high-performance fast-charge batteries.
1)Electrode materials Selecting suitable electrolytes and electrode materials to achieve high specific capacity and high rate performance has always been a challenging problem in battery design. Currently, many studies have been devoted to the development of fast-charging negative electrode materials without dendrites, such as carbon-based materials, metal oxide composites and alloys, which have achieved a certain degree of success. The potential of traditional graphite negative electrodes is very close to the redox potential of lithium, which can make the battery show a higher energy density, but at the same time increase the possibility of lithium precipitation. Therefore, improving negative electrode materials has become one of the important ways to improve the performance of lithium-ion batteries. In addition, LTO is considered to be promising for the development of long-life super-fast charging batteries because it does not precipitate lithium and does not form SEI film. On the other hand, LTO has a high potential, and as a negative electrode material it will reduce the voltage of the entire battery and limit the energy density of the battery. Some metal oxides and alloy materials also have good energy and power characteristics, but are limited by severe volume changes, pulverization and agglomeration, and their cycle stability is usually poor.
Other graphene-like two-dimensional materials have high surface area/mass ratio and unique physical and chemical properties, shorten the ion transport path, accelerate electron transport and increase lithium ion active sites, and are considered to be potential negative electrode materials. These materials mainly include transition metal oxides, transition metal sulfides, metal carbides and nitrides. Among them, the electrochemical window of titanium and niobium-based oxides is usually between 1.0-1.6V, which matches the current commercial electrolyte and is very suitable for negative electrode materials. Recently, the Goodenough team proposed a high-rate negative electrode material TiNb2O7 with a theoretical specific capacity comparable to that of graphite, and can achieve rapid lithium ion deintercalation and long cycle life, which is expected to replace LTO as a new negative electrode material.
Designing appropriate electrode structures at the nanoscale can also achieve high power and energy density, such as 2D hollow structures, core-shell structures, yolk-core structures, etc. Integrating 2D materials into macroscopic 3D structures can also enhance the transport of electrons and ions in the electrode.
Lithium metal is one of the negative electrode materials that can best improve the energy density of batteries, but its power performance is poor due to the low specific surface area of pure lithium metal foil. Introducing lithium metal into the 3D structural framework to accelerate the ion diffusion rate can significantly improve its rate performance. In addition to the selection, modification and nanostructure design of negative electrode materials, the electrode/electrolyte interface will also greatly affect the performance of negative electrode materials. The growth of lithium dendrites can also be inhibited by optimizing the negative electrode/electrolyte interface, such as amorphous carbon coated graphite to form a uniform SEI film, and selecting suitable lithium salts and co-solvents.
The selection and modification of materials will undoubtedly be the focus of future research. Compared with current commercial materials, many new materials show better fast charging performance. However, these materials are in the early stages of development and there is still a long time before large-scale commercialization. In many cases, issues such as developing new production processes and equipment and reducing costs may also hinder the application of new materials. In addition, the evaluation of many new materials and new structural designs only remains at the laboratory level. When they are applied to commercial batteries or packs, the actual effect may be greatly reduced. Materials science will undoubtedly play an important role in the development of future batteries, but a lot of engineering efforts are also needed to truly solve the problem of fast charging.
2)Battery cell and battery pack design In addition to material selection and microstructure design, the geometric parameters of electrode design also have an important impact on battery performance. Increasing the porosity and negative electrode thickness can inhibit lithium plating, but at the same time it will reduce energy density.
The capacity ratio (N/P) of the negative electrode to the positive electrode material significantly affects lithium deposition. In commercial lithium-ion batteries, N/P is often greater than 1. A higher N/P helps to reduce the mechanical stress of the negative electrode, reduce SEI formation and loss of active lithium. In NMC811/graphite batteries, the N/P ratio gradually decreases with the increase of the charge rate. This is because the surface capacity of graphite decreases more dramatically than that of NCM811 with the increase of the charge rate. The N/P ratio is 1.15 at 0.1C, 1.0 at 3C, and 0.5 at 4C. Studies have shown that during the static process after charging, the lithium metal precipitated in the main area of the negative electrode will diffuse to the protruding part of the negative electrode driven by the concentration gradient. In the subsequent discharge process, the edge of the positive electrode will receive more lithium accordingly. Continue to charge, and the excess lithium will be transferred to the negative electrode and the protruding area of the negative electrode corresponding to the edge of the positive electrode. This will lead to the phenomenon of increased local lithium concentration and decreased potential, increasing the possibility of lithium precipitation. Therefore, the protruding area of the negative electrode should be designed to be as small as possible to avoid lithium precipitation.
The geometric parameters of the battery are also important factors affecting the fast charging capability. The shape of the battery affects the distribution of current density and temperature. Large-sized batteries are more likely to cause uneven distribution of temperature and current. The position, material, structure and welding process of the tabs are very important for uniform distribution of current density, limiting local heat generation and delaying aging.
In addition, the relationship between battery pack performance and single-cell performance is not very clear. Although there are many fast-charging models for battery cells, few studies have attempted to expand them to pack design. This is because more parameters need to be considered when designing the pack. There are still many problems in the design of fast-charging battery packs: 1) Fast-charging packs require high performance of battery cells and low inconsistency between cells; 2) Monitoring and balancing of batteries requires advanced BMS with more sensors and circuit control; 3) Advanced thermal management systems need to be designed to maintain safe temperatures and reduce temperature differences within batteries and packs.
Fast charging strategy
Although many solutions at the material level have good effects, their commercialization is still difficult to achieve in the near future. Researchers have transferred the fast charging solution to the battery and pack level so that it can be applied in the short term. The design of the charging strategy is the key to solving this problem.
1) Types of charging strategies
Standard charging
CCCV is currently the most common charging protocol, which is to first charge at a constant current to the cut-off voltage (CC stage), and then charge at a constant voltage to a small current close to 0 (CV stage). The constant voltage process can make the ion concentration distribution in the electrode material more uniform, which is crucial for the material to exert a high specific capacity; but the current gradually decreases during constant voltage, making the charging time of CV significantly longer than CC. The simple operability of the CC-CV charging mode makes it the most widely used standard charging protocol. However, many other key strategies can reduce charging time, improve charging efficiency, and improve capacity/power retention. Figure 8 shows several common fast charging strategy curves.
Multi-stage constant current charging
Many studies have proposed that adjusting the current during the charging process can slow down battery aging and reduce charging time. The purpose of these studies is often to reduce heat generation, avoid lithium plating or reduce mechanical stress. MCC is one of the earliest strategies used for fast charging. It consists of two or more constant current stages, followed by a constant voltage stage. Since the negative electrode potential at the beginning of charging is not easy to drop to the lithium plating potential, the current in the early CC stage is relatively large. However, some researchers adopt the opposite charging strategy, that is, the current in the CC stage gradually increases, because the internal resistance of the battery will gradually decrease.
Pulse charging
During the pulse charging process, the current changes periodically to reduce concentration polarization, avoid local potential becoming negative, or reduce the increase in mechanical stress caused by local lithium ion insertion and extraction.
Enhanced charging
A larger average current is used in the initial charging phase, and then the current is reduced for CC-CV charging. The first charging phase can be a CC phase (the entire charging strategy is equivalent to MCC-CV), a CV phase (CV-CC-CV) after the battery voltage reaches the set maximum voltage, or a complete CC-CV phase (CC-CV-CC-CV). Compared to CC-CV, this strategy sets higher current and voltage to reduce the total charging time. However, under the same charging time, the capacity decay of enhanced charging is faster than that of CC-CV, and pulse charging is not significantly different from CC-CV. Some researchers have shown that CC-CV is suitable for fast charging of high-power batteries, and MCC is often used in charging scenarios where lithium is easily deposited.
Variable current charging
In order to achieve the purpose of fast charging, researchers have proposed a series of more complex variable current charging curves, including VCD, UVP, etc. As the battery ages, the current curve needs to be adjusted according to the change in internal resistance at the same voltage. In addition to the aging factor, the current is always very low in the initial charging stage, and then rises rapidly. This is because the internal resistance of the battery at 0% SOC is the largest, and then decreases rapidly. The maximum current often occurs in the lower SOC region, and then the current gradually decreases due to the increase in the amount of lithium embedded in the particles and the limited transmission of Li. In addition, the distribution of temperature inside the battery and the Pack during the charging process is very important, but the charging control strategy often only considers the surface temperature as the main attenuation factor.
Schindler combined the different charging strategies in Figure 9 to conduct cycle experiments on the battery, and compared it with CC-CV to study the capacity decay of the battery under different cycles. The results show that when combined with all charging strategies for cycle experiments, the battery maintained 80% of its capacity after 800 cycles, which was the best performance among all cycles; the battery with only CC-CV cycle decayed to the same capacity in only 400 cycles; and under the cycle combined with CC-CV and cold derating, the battery only cycled 330 times, which was the worst performance.
Figure 9 Current curves: a) AC pulse; b) cold derating; c) polarization retention; d) pulse charging
Most fast charging strategies are only effective at standard temperatures and specific battery configurations. Since large currents can cause greater mechanical stress inside the electrode particles, accompanied by significant current and temperature uneven distribution, caution should be exercised when fast charging is used for different types of batteries. The universality of many current charging strategies lacks further experimental verification. With the promotion of electric vehicles in low-temperature areas, more research on fast charging strategies under low temperatures is needed. In addition, it is the battery's own temperature rather than the ambient temperature that determines its performance, and changes in battery temperature during the charging process also need to be considered. Finally, the impact of different charging strategies at the Pack level needs to be studied urgently.
2) Model-based strategy optimization
Fast charging strategy based on ECM model
Some researchers have optimized charging strategies based on equivalent circuit models, and they use formulas to embed these models into single-objective or multi-objective optimization constraint problems. In these problems, first-order or higher-order equivalent circuit models are used to describe battery behavior, and multiple cost functions are set to achieve maximum charging efficiency or minimum charging loss.
Based on the equivalent circuit model, a thermal-electric-aging coupling model is established to describe the thermal effect or battery aging caused by charging, and the fast charging optimization of battery heating and aging can be performed based on the model. In addition to the commonly used lumped models, some enhanced models can separate the temperature inside and on the surface of the battery, or improve the simulation accuracy at high rates. Combining the charging rate, activation energy, total discharge capacity and temperature, the Arrhenius formula can be used to accurately simulate the aging phenomenon.
Once the framework of the optimization problem is established, a suitable algorithm can be developed for fast charging control based on the cost function and constraints. Common algorithms include: dynamic programming, Pontiac minimum principle, genetic algorithm, LGR pseudo-spectral method and minimum-maximum strategy.
The equivalent circuit can describe the external characteristics of the battery, but it cannot provide information about its internal state, especially the side reactions during charging, such as SEI film thickening, lithium deposition, etc. Therefore, electrochemical models have received attention.
Fast charging strategy based on electrochemical model
Electrochemical models can estimate the internal state of the battery (solid/liquid potential, ion concentration, reaction flow, etc.) to predict side reactions during charging. The most commonly used electrochemical model is the P2D model proposed by Doyle, Fuller, and Newman. However, in the full-order model (FOM), the computational complexity of solving the partial differential equation (PDE) is very large. Therefore, researchers have done a lot of simplification work based on FOM to increase the calculation rate. Some models also add side reactions to more realistically simulate the internal conditions of the battery. In recent years, some physically meaningful ECMs can also be used to describe the electrochemical processes inside the battery, and their parameter identification is simpler than P2D.
In summary , model-based optimization charging optimization usually gives priority to ECM, SP, ROM, etc. rather than FOM, because the former has less computational complexity and is more suitable for real vehicle applications. However, this usually comes at the expense of accuracy, so careful verification is required in certain abuse conditions such as fast charging simulation. Although there are many model-based optimization methods, few model results can completely match experimental data, and these matches are only applicable to fresh battery scenarios. The problem of establishing a long-term battery aging model needs to be solved urgently.
6.Impact of thermal management
Fast charging is often accompanied by a lot of heat generation and uneven heat generation. High-rate charging at low temperatures is very harmful to battery life and safety. Therefore, effective thermal management is very important to achieve lossless fast charging under all conditions. The design of the battery thermal management system will vary greatly at different temperatures. High thermal conductivity is required when cooling the Pack, while at low temperatures the Pack needs better thermal insulation to keep itself hot enough. Adjusting thermal conductivity according to temperature is one way to solve the problem.
1)Cooling
Common cooling media for electric vehicle Packs include air, liquid, and phase change material (PCM). The air cooling system is low-cost and simple, but due to its low heat capacity and poor thermal conductivity, the air cooling rate and temperature consistency are poor, and it is not suitable for fast charging systems. The cooling efficiency of liquid is 3,500 times higher than that of air, but its cost is high, the system is complex, and there is a possibility of leakage. To avoid short circuits, the cooling medium must be an insulator. Commonly used liquids include deionized water and mineral oil. PCM cooling uses the phase change process of the material to absorb the heat generated by the battery, but its disadvantages are also obvious: when the room temperature is very high, the PCM will completely melt even if the battery does not generate heat, and the liquid PCM with low thermal conductivity will hinder the heat dissipation of the battery.
Since fast charging will inevitably further aggravate the uneven temperature distribution, efficient and uniform cooling technology is more important than standard charging. The thermal conductivity of the battery interior is worse than that of the surface, and the battery surface is usually connected to the cooling system. These factors exacerbate the uneven distribution of temperature inside and outside the battery. Similar problems also occur in battery modules and packs.
Finally, some electric vehicle charging stations will configure corresponding external cooling systems according to charging conditions while improving the fast charging rate. If feasible, this approach will reduce the cost of the vehicle cooling system.
2)Preheating in low temperature environment
Low-temperature fast charging of lithium-ion batteries is very difficult. This section only introduces methods for quickly heating the entire battery, because fast heating is indispensable for fast charging. Internal heating methods are favored for their high efficiency and high uniformity. The four common methods are: 1) Self-discharge heating. This method is less efficient; 2) Battery-driven heating wire and fan heating. This method heats relatively quickly but is not efficient enough and the heating is uneven; 3) Bidirectional pulse heating. That is, a battery pack is divided into two groups of batteries with equal capacity, and the power is pulsed between the two groups of batteries, and the internal resistance is used for heating. This method is highly efficient and is mainly limited by DC/DC conversion. Simulation results show that this method can heat a 2.2Ah 18650 battery from -20℃ to 20℃ within 120s; 4) AC heating. This heating method is faster, but its impact on battery aging and cycle stability is still unclear. Designing the lithium-ion battery configuration to achieve fast preheating is also one of the ways to solve low-temperature fast charging. For example, an electrochemically separated nickel foil can be inserted between two single-sided negative electrode layers, and a direct current can be controlled by a switch to flow through the nickel foil for rapid heating.
Although internal heating methods are more effective and result in more uniform temperature distribution, the effects of coupling internal heating with fast charging on battery cycle life are poorly studied. Since current flows more easily through low-resistance areas, the temperatures in those areas rise, so even small temperature gradients caused by preheating are amplified during fast charging. Since internal temperatures are difficult to measure experimentally, cycle tests or reliable models are needed to evaluate preheating methods. Although nickel foil preheating is promising, it requires nonstandard battery design and adds weight, as well as other possible issues.
7.Security
1)Impact of fast charging on thermal runaway
Studies have shown that the thermal runaway behavior of batteries changes after fast charging. For example, after ARC testing of high-energy soft-pack batteries after fast charging, it was found that the thermal runaway temperature of the battery after fast charging was significantly lower than that of fresh batteries, and these effects can be eliminated if there is enough standing time. As the standing time increases, the precipitated lithium gradually re-embeds into the negative electrode, and part of the lithium reacts with the electrolyte to form a new SEI film. Therefore, the lithium involved in the thermal runaway process will decrease, and the thermal runaway characteristics of the battery will gradually return to the level of fresh batteries.
Thermal runaway is caused by a series of chain reactions. Thermal runaway of a fresh battery is usually caused by a short circuit, followed by a reaction of the electrolyte, and the battery temperature reaches the highest. The thermal runaway process of the battery after fast charging can be divided into three stages, as shown in Figure 10. In the first stage (60℃ < T< 110℃), the precipitated lithium reacts with the electrolyte to heat the battery, and the SEI film is constantly ruptured and regenerated. At this time, the temperature is relatively low. In the second stage (thermal runaway induction stage), a large amount of lithium metal is consumed in the reaction with the electrolyte, causing a sharp increase in temperature. The diaphragm shrinks and the positive and negative electrodes come into contact. In the third stage (thermal runaway to the highest temperature), due to the sudden increase in temperature, the positive and negative electrodes begin to react with the electrolyte and between the positive and negative electrodes. Finally, the battery temperature reaches the highest and thermal runaway occurs.
Figure 10 Chain reaction of battery thermal runaway process after fast charging
2)Thermal runaway caused by overcharging
Some fast-charging battery packs may be overcharged due to inconsistencies between battery cells, which may lead to thermal runaway. This process can be divided into four stages:
Stage 1 (100% < SOC < 120%): The voltage starts to increase slowly after exceeding the charge cut-off voltage. At this time, the excess negative electrode material can still insert lithium normally to ensure safety. Some side reactions of the battery material may be induced, and the battery internal resistance and temperature increase slightly.
Stage 2 (120% < SOC < 140%): Due to excessive delithiation, transition metal ions such as Mn2+ in the positive electrode begin to dissolve. At the same time, because the voltage exceeds the electrochemical window of the electrolyte, the electrolyte also begins to oxidize. The negative electrode cannot receive more lithium ions and begins to become unstable and lithium precipitation occurs. The lithium metal reacts with the electrolyte to form a new SEI film, increasing the internal resistance of the battery. The Joule heat generated by overcharging will significantly increase the battery temperature.
Stage 3 (140% < SOC < 160%): The exothermic reaction of the battery material quickly catches up with and exceeds the Joule heat generated by the current, and becomes the main heat generation method. The electrolyte oxidizes to generate a large amount of heat and is accompanied by gas production, causing the battery pack to swell. As the amount of lithium precipitated increases, its side reaction with the electrolyte becomes more intense. When the SOC approaches 160%, a large amount of Mn2+ in the positive electrode dissolves. The positive electrode structure begins to change, the battery voltage reaches a maximum value and begins to gradually decrease.
Stage 4 (140% < SOC < 160%): The electrolyte decomposes and produces a large amount of gas, causing the battery pack to rupture suddenly. The diaphragm ruptures, a large-area internal short circuit occurs inside the battery, and the battery eventually goes into thermal runaway.
Based on the internal materials and reaction kinetics of the battery, two design methods have been proposed to protect the battery from overcharging:
1)Raising the oxidation potential of the electrolyte from 4.4 V to 4.7 V can make the electrolyte more stable and increase the SOC at which thermal runaway occurs to 183%. This can be achieved by adding functional additives or additives that can undergo reversible redox reactions to the electrolyte.
2)The battery thermal runaway temperature is raised to 300°C to delay the occurrence of large-area internal short circuits, and the SOC at which thermal runaway occurs is increased to 180%. The rupture of the battery pack can be delayed by optimizing the battery pressure design or using a diaphragm with high heat exchange stability.
8. Conclusion
The electrification of transportation is undoubtedly one of the important means to solve climate change. In order to deal with range anxiety and meet customer needs, many manufacturers regard the fast charging capability of the Pack as an important indicator. Although there have been many studies on fast charging in recent years, there are still many problems:
1.To date, there is still no reliable on-board method to detect battery aging (such as lithium plating and mechanical rupture). Lithium plating detection methods based on voltage platforms are expected to be applied online, but there is no relevant research on how to effectively distinguish lithium dissolution platforms from other phenomena, and detect lithium plating without voltage platforms.
2.Many new electrode materials have good fast charging capabilities, but their stability, attenuation mechanism, large-scale production and cost are still under discussion. Although graphite anode is very easy to precipitate lithium, considering the cost, wide application and maturity of technology, graphite will occupy the main market of lithium-ion battery anode materials in the foreseeable future.
3.Existing model methods have obvious limitations: ECM-based models cannot predict the internal state of the battery and can only be used within a limited range. On the other hand, the high-precision FOM model cannot be applied in real time due to the large amount of calculation. Therefore, a reduced-order model is needed to accurately describe the internal core state of the battery for application in future fast-charging BMS systems.
4.Many fast-charging strategies are developed based on experience or experiments, and their results are only applicable to batteries of specific configurations or specific operating conditions, and cannot be extended to other types of batteries. In addition, many model-based charging optimization studies are based on SP or ECM models, and the model prediction accuracy is often insufficient under high currents.
5.There is currently little research on strategies for optimizing fast charging at low temperatures, which is crucial for the promotion and application of electric vehicles in cold regions.
6.In order to further optimize the charging process of single cells in the battery pack and avoid local aging or overcharging, it is also necessary to develop an advanced BMS system with balanced battery consistency.
7.Although a lot of research has been devoted to the design of thermal management systems, the efficiency and uniformity of various preheating and cooling systems still need to be evaluated in depth. Almost no researchers have evaluated the impact of AC preheating coupled with fast charging on battery life. Optimizing the design of the tabs, the location and geometry of the cooling system are also important means to improve the uniformity of temperature and current. External cooling technology coupled with charging piles is also an important way to reduce the cost and quality of the vehicle cooling system, but its true effect remains to be further observed.
8.Finally, the relationship between the attenuation rate of battery cells and packs is still unclear. Although many charging and preheating strategies are effective on battery cells, their effectiveness, feasibility and cost in the pack are still lacking. Some effective charging strategies for single cells may cause uneven temperature and current density distribution when applied to the pack, so any non-traditional charging technology requires a lot of research before practical application. In addition, almost no model considers the impact of inconsistencies between cells within the battery pack. Since fast charging will amplify inconsistencies, multi-size research is urgently needed. Multi-size research is crucial for battery cell integration and pack design.
Summary of laminated lithium-ion battery design (capacity, structure, pole piece, diaphragm, electrolyte volume, etc.)
1. Design capacity
In order to ensure the reliability and service life of the battery design, the design capacity is determined based on the minimum capacity required by the customer .
Design capacity (mAh) = required minimum capacity × design factor (1)
(1)The battery design factor is generally 1.08±0.01, and 1.06 can be used when the difficulty is high;
(2)The design coefficient of the extended capacity battery should be ≥1.04
2. Structural design
2.1 Design of membrane cavity length
The length of the membrane cavity and the length of the battery cell have the following relationship:
Membrane cavity length = cell length - A (2)
A — coefficient, the value is determined by the thickness T of the battery cell.
(1) When T≤3mm, the value of A for conventional battery cells is generally 4.5mm, and the value of A for large battery cells is generally 4.8mm;
(2) When 3mm<T≤4mm, the value of A for conventional battery cells is generally 4.8mm, and the value of A for large battery cells is generally 5.0mm;
(3) When 4mm<T≤5mm, the value of A for conventional battery cells is generally 5.0mm, and the value of A for large battery cells is generally 5.2~6.0mm;
(4) When 5mm<T≤6mm, the value of A for conventional battery cells is generally 5.2mm, and the value of A for large battery cells is generally 5.4~6.0mm.
2.2 Membrane cavity width design
The width of the membrane cavity and the width of the battery cell have the following relationship:
Membrane cavity width = cell width - B (3)
B—coefficient, generally 1.0~1.2mm (applicable to double folding).
2.3 Groove depth design
The groove depth H of the aluminum-plastic packaging film is determined according to the theoretical stacking thickness T' of the battery cell. In principle, the groove depth is equal to the thickness of the battery cell after stacking.
T'= T positive + T negative + T diaphragm
= hpositive × Npositive + 2hsingle + hnegative × Nnegative + hdiaphragm ×(Nnegative + 1)×2 (4)
H = T' ± 0.1 ( 5 )
Note: The thickness after rolling determined by the surface density in the above formula should be determined according to the lower limit of the compaction density of the corresponding material, that is, the thickness of the laminate should be calculated using the upper limit of the rolling thickness.
in:
Tpositive – total thickness of the positive electrode sheet ;
Tnegative – total thickness of negative electrode sheet ;
T Diaphragm - the total thickness of the diaphragm after stacking into a battery cell. The thickness of the diaphragm is generally 0.020/0.016mm;
hpositive - the thickness of the positive electrode sheet ( double -sided) after rolling;
h single - the thickness of the positive electrode single-sided sheet after rolling;
h negative - the thickness of the negative electrode sheet (double-sided) after rolling;
N negative - the number of negative plates;
hDiaphragm — thickness of the diaphragm .
3.Pole piece size design
Determine the length and width of a single electrode according to the size of the battery to be designed.
Pole length Lp:
Lp = membrane cavity length - C (6)
Pole width Wp:
Wp = cell width - D ( 7 )
The length of the tail pole piece Lp′:
Lp′= 2Lp+T'-1.0 (8)
Width Wp′ of the tail pole piece:
Wp′= Wp-0.5 (9)
Where: C — clearance coefficient, generally ranging from 3.6 to 4.0 mm;
D — The value range is generally 2.5~2.6mm (applicable to double folding);
T'— Theoretical stacking thickness of the battery cell.
Figure 1. Schematic diagram of double-sided pole piece and single-sided positive electrode package tail pole piece
4.Determination of pole piece number and surface density:
Determine the number of pole pieces N, and determine the surface density of the electrode according to the design capacity of the battery. The design capacity of the battery is generally determined by the positive electrode capacity, and the negative electrode capacity is in excess. When performing theoretical calculations, the mass specific capacity of the positive electrode active material is generally 140 mAh/g, and the mass specific capacity of the negative electrode active material is 300 mAh/g.
N = (T-0.2)/0.35±1 (10)
Note: N is rounded up during calculation and adjusted according to the surface density value calculated by formula (12). The double-sided surface density of the positive electrode of a conventional battery should be ≤420g/m 2 , and the double-sided surface density of the negative electrode should be ≤200g/m 2 .
S pole piece = Lp×Wp (11)
C = C proportional × S pole × N × ρ positive × η positive (12)
Cnegative = Cset ×υ ( 13)
= C negative ratio × S pole piece × N × ρ negative × η negative (14)
in:
S pole piece - the area of a single pole piece;
C proportional - the mass specific capacity of the positive electrode active material, generally 140mAh/g;
ηpositive - percentage of positive electrode active material ;
ρpositive - double - sided surface density of the positive electrode (g/m 2 );
C negative – the design capacity of the negative electrode;
υ — excess capacity coefficient of negative electrode, generally the value of conventional battery is 1.04±0.02; the value of DVD battery and battery with capacity greater than 2000mAh is generally 1.07±0.01;
C negative ratio - the mass specific capacity of the negative electrode active material, generally 300mAh/g;
η negative - percentage of negative electrode active material;
ρnegative - double-sided surface density of negative electrode (g/m 2 );
5. Determination :
In order to ensure the performance of the active materials in the electrode, the electrode should be properly rolled after coating, and the rolling thickness of the electrode with different surface density should be determined according to the compaction density of the material.
Table 1. Compacted density of positive and negative electrode materials
Material | Compacted density /g/ m3 |
Cathode Materials | 3.70±0.20 |
Negative electrode material BF | 1.55±0.10 |
Negative electrode material MCMB | 1.70±0.10 |
6.Determination of diaphragm size
The specifications of the diaphragms currently used are generally 0.020mm and 0.022mm in thickness . The length Ls and width Lt of the diaphragm are determined by the following formula:
Ls = (Wp+0.5)×(2×N+2) (15)
Lt = Lp +Ψ 16 )
Where: Ψ — The width of the diaphragm exceeds the length of the pole piece, generally 3.0mm.
7.Determination of the amount of electrolyte
Determine the amount of electrolyte added according to the design capacity of the battery
M = C ÷ ξ (18)
in:
ξ— generally 270mAh/g.
8.Tab selection
Determine the size of the tabs based on the width and capacity of the battery, and refer to the table below for selection.
Table 4 Relationship between battery size, capacity and tab size
Battery width/mm | Capacity/mAh | Ear width/mm |
<20 | —— | 2.0 |
≥20 | <1200 | 3.0/4.0 |
≥1200 | 5.0 |
9.Lamination thickness design calculation formula
According to the formula, whether the thickness of the battery cell meets the customer's requirements can be determined by referring to the following formula:
Finished cell thickness T < positive electrode pressing thickness × ( number of stacking pairs - 1) + negative electrode pressing thickness × number of stacking pairs + positive electrode single-sided thickness × 2 + (number of stacking pairs × 2 + 2) × diaphragm thickness + number of stacking pairs × expansion coefficient + packaging film thickness × 2
The expansion coefficient is as follows
Positive electrode surface density | Expansion coefficient |
360~380 g/ m2 | 0.050mm |
381~400 g/ m2 | 0.055mm |
401~420 g/ m2 | 0.060mm |
Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials, electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email: contact@canrd.com Phone/Wechat/WhatsApp: +86 19867737979
Canrd Official Web Canrd Company Vedio Canrd Company profile
Website : www.canrud.com





































No comments:
Post a Comment