1. Key Factors Influencing Formation: Mechanism
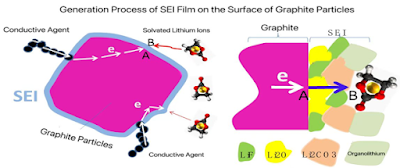
Generation Process of SEI Membrane:
l Electrons are transferred from the current collector, through the conductive agent, to point A inside the graphite particles where the SEI membrane is to be formed.
l Solvated lithium ions, wrapped in the solvent, diffuse from the cathode to point B on the surface of the SEI membrane that is currently being formed.
l The electrons at point A diffuse to point B through the electron tunneling effect.
l The electrons that jump to point B react with lithium salt, solvated lithium ions, film-forming agents, etc., to continue generating the SEI membrane on the surface of the existing SEI membrane. This process results in the continuous increase of the SEI membrane thickness on the surface of the graphite particles, ultimately leading to the formation of a complete SEI membrane.
Two-electron reaction:
l This type of reaction requires the participation of two electrons simultaneously. Under such conditions, it is easier to form inorganic lithium salt components.
l One-electron reaction: This reaction occurs with the participation of only one electron. In this case, it is more likely to generate organic lithium salt components.
2. Key factors influencing the formation process
l Key factors influencing SEI film composition:
n Electron density
n Concentration of reactants
l Which process parameters affect electron density and reactant concentration?
n Electron density:
u Current magnitude
u Pressure (applied between the positive and negative electrodes)
l Reactant concentration:
n Temperature
n Composition and concentration of electrolyte
l Others:
n Formation cutoff voltage
2.1. Formation current
Let the formation current be I:
l Electrons are transferred from the current collector to the conductive agent and then into the interior of the graphite particles to point A, where the SEI film is to be formed.
l For a flat interface: The number of electrons reaching point A will be determined by the formation current I. The larger the formation current, the greater the current passing through point A on the electrode.
l Different current densities will produce SEI films with different structures.
2.2. Formation Pressure (Current Uniformity)
Formation pressure P:
l When the interface is not flat, the current distribution at the electrode interface is uneven, with a higher current density at points (a) that are closer together and a lower current density at points that are further apart. This uneven current distribution leads to an inhomogeneous SEI film structure.
By applying a pressure of P, the electrode interface can be flattened, enabling a uniform distribution of current across the interface and the formation of a uniform SEI film.
2.3. Formation Temperature
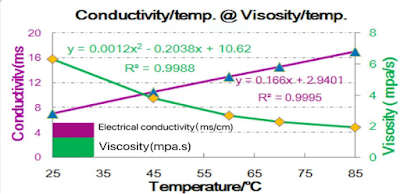
l For the same electrolyte, increase the temperature, the viscosity of the electrolyte decreases, reducing the resistance to the transport of reactants (film-forming agents/lithium ions) within the electrolyte.
l For the same electrolyte, higher temperature leads to an increase in electrical conductivity and a decrease in polarization, further reducing the resistance to the transport of reactants in the electrolyte.
l More film-forming agents and solvated lithium ions can reach point B within a given time, thereby influencing the SEI film-forming reaction.
2.4. Composition and Concentration of Electrolyte
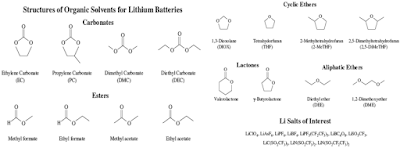
Electrolyte Components:
l A wide variety of electrolyte solvents, additives, and salts.
l Different electrolyte components participate in different film-forming reactions, resulting in differences in the composition of the SEI film formed.
2.5. Formation Cutoff Voltage
Formation Cutoff Potential: The electrochemical windows of various components can be obtained through CV testing:
l Different electrolyte components have different electrochemical windows.
l There are also subtle differences in different anode-cathode systems.
3. Pouch Battery Formation Process Parameters
Key Parameters
Ø Charge 1/Charge 2 rate
Ø Formation Temp,
Ø Pressure,
Ø SOC (Time),
Remark:
Ø Heating/Formation in the Same Fixture
Ø Charge 1: Form SEI
Charge 2: OCV/THK/ripping
3.1. Charge Current 1
Current of step 1→form SEI
l Current ↗, reaction peak →, polarization↗, effect SEI form
l All data of step 1 use 05 or 1C and 02C is in the same level by now.
Suggested formation current of 1st step =0.5~1.0C
3.2. Charge Current 2
Current of step 2→for Charging
l Current ↗, polarization↗.
l 2C of step 2 is OK.
Suggested formation current of 2nd step =1.0 ~ 2.0C
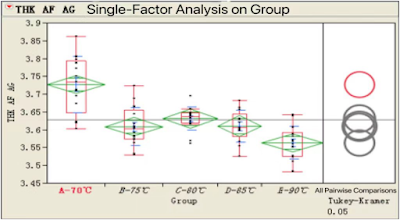
3.3. Formation Temperature
Temp.=90℃, D0 ↘;
Suggested formation Temp = 70~85 degC
3.4. Formation Pressure
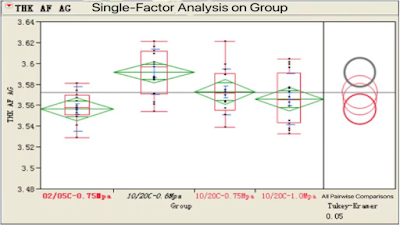
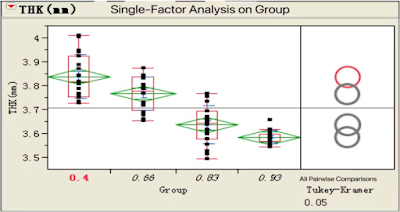
Pressure↗, THK ↘ af. Cap
Suggested formation pressure : 0.6~1.0 MPa
3.5. Formation SOC
l SOC↗,THK ↘,
l For rippled cell, High SOC is a must
Suggested formation SOC = 66%~83%
4. Coin Cell Formation
Key Parameters: Typically focused on performance with less attention paid to efficiency.
l Charge 1/Charge 2 Rate: Low-current charging (0.02C).
l Formation Temp: Room temperature (some laboratories may adopt 45℃ under certain conditions).
l Pressure,Double gasket structure is used for coin cell assembly, with high flatness and rigidity of the gaskets to maintain a flat interface.
l SOC (Time),A complete charging (cathode/full cell) or discharging (anode) process.
4.1. Determination Method 1 for Formation Current/Cutoff Potential
Remarks:
l Lithium cobalt oxide/graphite system;
l Electrolyte: Electrolyte with VC and FEC as additives;
l dQ/dV curve, similar to CV curve; with voltage as the horizontal axis and the integral of voltage with respect to energy as the vertical axis;
4.2. Determination Method 2 for Formation Current/Cutoff Potential
l Method for Determining Formation Current and Cutoff Potential: Trial-and-Error Method.
l Experimental Process:
n Conduct constant-current charging at 0.02C until the battery is fully charged (4.2V). Calculate the dQ/dV value and plot a dQ/dV-V curve. The position of the first reaction peak (~2.8V) represents the potential at which the film-forming additive decomposes to form the film. The formation cutoff potential is ~2.92V.
n Select a current of 0.03C to form parallel samples. Obtain the position of the first reaction peak (~2.8V), which represents the potential at which the film-forming additive decomposes to form the film. The formation cutoff potential is ~2.94V.
n Select a higher current (0.05C) to form parallel samples. Obtain the position of the first reaction peak (~2.9V), which represents the potential at which the film-forming additive decomposes to form the film. The formation cutoff potential is ~3.16V.
l Experimental Conclusion:
n When forming at 0.02C and 0.03C, the positions of the film-forming reaction peaks overlap, indicating that the entire SEI film-forming reaction is the same. When forming at 0.05C, the peak position shifts significantly to the right, indicating increased polarization, which will affect the SEI film-forming effect. Therefore, from the perspective of saving formation time and obtaining battery cells with excellent performance, selecting a formation current of 0.03C is a preferable result.
n When forming with different formation currents, the cutoff potentials for the film-forming reaction are 2.92V, 2.94V, and 3.16V, respectively. Therefore, we can select a formation cutoff potential of 3.5V. In other words, the formation process is 0.03C CC to 3.5V.
5. Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials, electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email: contact@canrd.com Phone/Wechat/WhatsApp: +86 19867737979
Canrd Official Web Canrd Company Vedio Canrd Company profile
Website : www.canrud.com
6. Q & A
In this Q&A session, Dr. Ke also answered all the questions raised by everyone.
Kite without a string: "What does the current density affect?"
Dr. Ke: "The current density affects the electron concentration at the reaction interface. A higher electron concentration makes it easier for a two-electron reaction to occur."
Student Xiao Peng—NCM811: "What impact does ambient temperature have? What are the drawbacks of too high or too low temperatures?"
Dr. Ke: "We will talk about the specific influence of single factors on formation later. For now, we are mainly discussing the theoretical basis of formation process parameters. Let's see if anyone has any comments or suggestions."
Kite without a string: "Why is formation always done with a small current?"
Dr. Ke: "That description is not accurate. Once we cover the formation process parameters, you'll find that it's not always done with a small current."
Crayon Little Pig☠: "What happens to CV outside the electrolyte window?"
Dr. Ke: "There will be a strong irreversible reaction peak."
Let youth continue: "Dr. Ke, what impact do different material systems have on the formation process?"
Dr. Ke: "That's a very broad question. Mainly, the electrolyte affects the formation process. You can refer to the PPT slides on electrolyte composition and the cutoff voltage for formation."
Hei Hei: "Was the formation thickness limit mentioned just now 3.7?"
Dr. Ke: "That depends on the thickness of the experimental cell. There's no fixed limit."
Weirong Supercapacitor: "We are using a new material, and all the parameters are unknown. How can we determine what 0.1C is?"
Dr. Ke: "You can use a very small current (0.01C) to perform a charge-discharge cycle, and you will know the exact capacity. (Of course, you should have a rough estimate of the capacity of your material)."
Kite without a string: "Is high-temperature formation mainly to improve efficiency?"
Dr. Ke: "It can also improve the consistency of the SEI (because polarization is reduced), thus improving battery performance."
West China University of Technology: "Dr. Ke, should the film formed at high temperatures be relatively loose?"
Dr. Ke: "There are specific studies on the impact of temperature on SEI composition. The conclusion is that high temperatures can convert the organic components of the SEI into inorganic components."
Kite without a string: "Does the current size also affect the compactness of the SEI?"
Dr. Ke: "We discussed the impact of current size on formation results in the first part. The current size affects the reaction equations, thus influencing the SEI composition."
Angel not online: "Can formation pressure control thickness? Will it rebound?"
Dr. Ke: "After releasing the pressure post-formation, there will be some rebound in thickness. This is normal, but monitoring is always done on the finished battery thickness."
Angel not online: "How is the cutoff voltage for formation determined?"
Dr. Ke: "Mainly by the peak position of the film formation reaction, and to be safe, the cutoff voltage is slightly increased."
A little bit: "Is there any way to improve self-discharge?"
Dr. Ke: "Self-discharge is a systems engineering issue. Materials, design, and process are all closely related."
Angel not online: "Do we need to reselect points for the dQ/dV vs. V graph?"
Dr. Ke: "You can process the data from the charge-discharge tester."
Angel not online: "Is the formation for full-cell soft pack done with a single constant current charge to the cutoff voltage?"
Dr. Ke: "Yes, constant current cutoff."
Sodium/Potassium battery: "Could I ask a question? What is the specific temperature for high-temperature formation?"
Dr. Ke: "The current high-temperature formation temperature for lithium-ion batteries ranges from 75°C to 85°C."




















No comments:
Post a Comment