1. Basic Concepts of Formation
1.1. What is Formation?
l Formation refers to the process of activating the cathode and anode materials inside a battery after it has been fully rested following electrolyte injection. This activation is achieved through a specific charging and discharging cycle, which also leads to the formation of a SEI (Solid Electrolyte Interphase) film on the surface of the active materials. The SEI film helps to improve the overall performance of the battery in terms of charging and discharging, self-discharge, and storage capabilities.
l Formation is the initialization process for lithium-ion battery cells, which activates the active materials within the cell. Its most substantial content is the formation of the SEI film. (The SEI film is a passivating thin layer that forms on the surface of the negative electrode during the first charge when Li+ ions detach from the positive active material and arrive at the graphite of the negative electrode. )
l The benefits of SEI film: stabilizing the electrode active materials and preventing the electrolyte from continuously reacting with the active materials in an irreversible manner. Therefore, the quality of the SEI film directly determines the electrochemical performance of the battery, including its capacity, rate capability, cycle life, and performance under high and low temperatures.
The drawbacks of SEI film: It consumes a limited amount of lithium ions from the battery, necessitating the use of more lithium-containing cathode materials to compensate for the lithium loss during the initial charging process. Additionally, the SEI film increases the resistance at the electrode/electrolyte interface, causing a certain voltage lag.
2. Mechanism of SEI Film Formation During the Formation Process
2.1. Formation Mechanism of SEI Film
l The process of SEI film formation:
n Under a certain potential, the reactant components (such as Li+, lithium salt, additives, solvents, etc.) gather at the interface between the electrode material and the electrolyte phase.
n Under a certain potential, electrons diffuse to the interface between the electrode material and the electrolyte phase.
n The reactant components and electrons undergo irreversible reactions at the interface, leading to the formation of SEI.
n These irreversible reactions mainly occur during the first charging process of the battery.
n Once the electrode surface is completely covered by the SEI film, the irreversible reactions stop.
n Once a stable SEI film is formed, the battery can undergo multiple stable charging and discharging cycles.
n During the battery's cycling and overcharging, the SEI film maintains a dynamic stable equilibrium state, with decomposition and formation reactions occurring simultaneously.
l Structure and Composition of SEI Film:
n The thickness of SEI film is approximately 100~120nm,
n Inorganic Components: Li2CO3, LiF, Li2O, LiOH, etc.
n Organic Components: ROCO2Li, ROLi, (ROCO2Li)2, etc.
n Both alkyl lithium carbonate and Li2CO3 are the main components that form the SEI film before 3.5V.
n After 3.5V, alkyl lithium carbonate and alkoxylithium become the primary components of the SEI film.
Note: SEI film can also form on the surface of the cathode material, but it is currently believed that its impact on the battery is much smaller than that of the SEI film on the surface of the anode. Therefore, this article focuses on discussing the SEI film on the surface of the anode (all subsequent mentions of SEI film, unless otherwise specified, refer to the one formed on the anode).
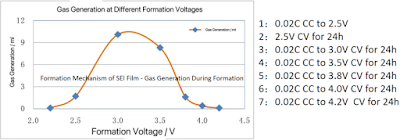
2.2. Formation Mechanism of SEI Film - Gas Generation During Formation
l Battery Design Information:
n Battery Capacity: 1300mAh;
n Cathode/Anode/Separator: 4.2V Lithium Cobalt Oxide / Synthetic Graphite / PP Separator
n Electrolyte: 1mol/L LiPF6 EC:DMC:EMC=1:1:1;
l Experimental Procedure:
n The gas generation volume was measured using a water displacement method: the battery cell body was soaked in water with the tabs exposed above the surface, and then charged;
n The charging cutoff voltages were set at below 2.5V, 2.5V, 3.0V, 3.5V, 3.8V, 4.0V, and 4.2V respectively;
l Experimental Conclusion:
n The total gas generation reached a maximum at 3.0V; after exceeding 3.5V, the amount of gas generated rapidly decreased.
2.3. Formation Mechanism of SEI Film - Composition of Gases Generated During Formation
l Components of Gases Generated at Different Cutoff Potentials (GC-MS Testing):
n During reactions at low voltages (<2.5V, 2.5V), the gases produced are primarily H2, CO2, and CO.
n During reactions at medium voltages (3.0V, 3.5V), the gases produced are mainly CO2, C2H4, and CO.
n During reactions at high voltages (3.8V, >3.8V), the gases generated are primarily CH4, C2H4, C2H6, C3H6, C3H8, and CO.
2.4. Detailed Explanation of Gas Generation During SEI Film Formation
l Interpretation of the Gas Generation Process:
n When the formation voltage is less than 2.5V, the gases produced are mainly H2 and CO2, but the gas generation volume is extremely small, and no obvious film-forming reaction.
n At a formation voltage of 2.5V, the EC in the electrolyte begins to decompose. When the voltage is within the range of 3.0~3.5V, the decomposition of EC accelerates, resulting in a large amount of C2H4.
n When the formation voltage exceeds 3.0V, the DMC and EMC in the electrolyte decompose, producing alkanes such as C2H4, CH4, and C2H6.
n After the formation voltage exceeds 3.8V, the reduction and decomposition of DMC and EMC become the primary reactions.
n Between the formation voltages of 3.0~3.5V, the amount of gas generated during the formation process is the largest, and the formation of the SEI film is most obvious. After the voltage exceeds 3.5V, the SEI film has basically formed, so the reduction and decomposition reactions of the electrolyte solvents are suppressed, and the amount of gas generated also decreases rapidly.
The formation range of the SEI film is between 3.0V and 3.5V, where the main component of the gas generated during formation is C2H4. Therefore, it can be concluded that the formation mechanism of the SEI film at this stage is mainly the reduction and decomposition of EC in the electrolyte solvent.
2.5. Formation Mechanism of SEI Film - Charging Chemical Reaction
Cathode Reaction: LiCoO2=Li1-xCoO2+xLi++xe-
Anode Reaction: 6C+xLi++xe-=LixC6
Overall Battery Reaction: LiCoO2+6C=Li1-xCoO2+LixC6
Voltage below 2.5V H2O+e→OH-+1/2H2 (g)
OH-+ Li+→ Li OH (s)
Li OH + Li+ + e→LiO(s)+1/2H2 (g)
LiPF6→LiF+PF5
PF5+H2O→2HF+PF3O
LiCO3+2HF→LiF+H2CO3
H2CO3→H2O+CO2(g )
2.6. Formation Mechanism of SEI Film - Chemical Reaction
l The main reactions during the formation of the SEI layer are as follows:
EC+ e →EC·(EC Radical )
2EC·+2Li+→CH2=CH2 (g)+(CH2OCO2Li)2 (s)
EC+2e→CH2=CH2 (g)+CO32-
CO32- + 2Li+→Li2CO3
EC+2Li++2e→CH3OLi (s) + CO (g)
DMC + e+ Li+→CH3OCO2Li (s)+CH3·
DMC+ e+ Li+→CH3OLi (s)+CH3OCO2
CH3OCO2+CH3·→CH3OCO2CH3
EMC+ e+ Li+→CH3OCO2Li (s)+C2H5·
CH3·+1/2H2→CH4
C2H5·+1/2H2→C2H6
CH3·+CH3·→C2H6
C2H5·+CH3·→C3H8
DMC+2Li++2e→CH3OLi (s) + CO (g)
l The gases generated by the chemical reactions during the formation process include H2, CO, CO2, C2H4, CH4, and C2H6. Therefore, there is always an airbag inside the battery cell during the formation process, the purpose is to store the gases generated.
l The quality and stability of the SEI film formation are crucial factors that cannot be ignored in determining the battery's capacity, initial efficiency, cycling performance, rate capability, and performance at high and low temperatures.
3. Deep Reflection on SEI Film-Related Issues
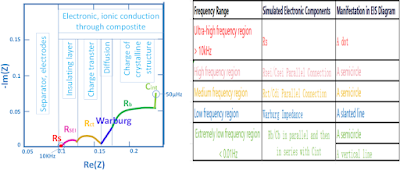
3.1. Question 1 - EIS Spectrum
3.2. Question 1 - Physical and Chemical Processes of Charging
l During the charging process, the desorption and intercalation of lithium ions in the intercalation electrode involve the following five steps:
n Step ①: The transmission of electrons to the surface of the active material and their transfer between active material particles, as well as the transmission of lithium ions in the electrolyte.
n Step ②: Diffusion and migration of lithium ions through the SEI film on the surface of active material particles.
n Step ③: The charge transfer process (or reaction binding process) between electrons and lithium ions at the conductive junction.
n Step ④: Diffusion of lithium ions within the solid particles of the active material.
n Step ⑤: Accumulation and consumption of lithium ions in the active material, leading to changes in the crystal structure of the active material particles or the formation of new phases.
3.3. Question 1 -The Relationship between EIS Spectrum and Physical and Chemical Processes of Charging
Remark:
Rsei represents the resistance of lithium ion diffusion and migration through the SEI film.
Csei represents the capacitance of lithium ion diffusion and migration through the SEI film.
Rct refers to the charge transfer resistance, also known as the electrochemical reaction resistance.
CdI stands for the double-layer capacitance.
Rb characterizes the resistance associated with changes in the bulk structure of active material particles.
Cb characterizes the capacitance associated with changes in the bulk structure of active material particles.
Cint represents the intercalation capacitance that characterizes the accumulation or consumption of lithium ions in the active material
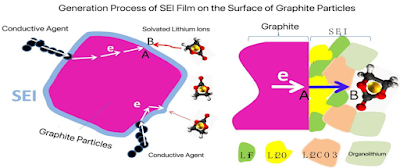
3.4. Question 2 - SEI Growth Process 1
l The formation process of the SEI film:
n Electrons are transferred from the current collector, through the conductive agent, to the interior of the graphite particles, ultimately reaching point A where the SEI film is to be formed.
n Solvated lithium ions, surrounded by solvents, diffuse from the cathode to point B on the surface layer of the SEI film that is currently being formed.
n The electrons at point A diffuse to point B through the electron tunneling effect.
n The electrons that have migrated to point B react with lithium salts, solvated lithium ions, film-forming agents, and other components, continuing to generate the SEI film on the surface layer of the existing SEI film. This process leads to a continuous increase in the thickness of the SEI film on the surface of the graphite particles, ultimately resulting in the formation of a complete SEI film.
For the SEI film formation reaction to occur, electrons must reach the surface of the already formed SEI film (point B). However, the SEI film itself is electronically insulating, allowing electron transport only through the electron tunneling effect. This limits the thickness that electrons can penetrate, thus determining that the SEI film cannot grow indefinitely in the direction of thickness.
3.5. Question 2 - SEI Growth Process 2
l Experimental Process (by Perla B. Balbuena et al.):
n Using highly oriented graphite as the research substrate and a lithium hexafluorophosphate + EC + DEC system as the electrolyte, the sample was prepared through the formation process.
n The method of argon atom bombardment sputtering + XPS was used to examine the composition of the sputtered material at different sputtering times.
l Result: Detected the existence of the SEI film
n The thickness of the SEI film on the end plane of the highly oriented graphite is approximately 30nm.
n The thickness of the SEI film on the base plane of the highly oriented graphite is approximately 2nm.
l Reason Analysis:
n During the charging and discharging process, the intercalation and deintercalation of lithium ions mainly occur at the end plane of the highly oriented graphite.
n A thicker and more structurally intact SEI film is required for protection on the end plane.
3.6. Question 3 - Composition of SEI
l Using PC as the solvent, ES as the additive, and 1M LiPF6 as the lithium salt to prepare the electrolyte, a battery was assembled with graphite as the active material to study the SEI film formation reaction. The research found that the main reaction processes include two categories:
n Two-electron reaction: This reaction occurs when two electrons participate simultaneously, and it is more likely to generate inorganic lithium salt components.
n One-electron reaction: This reaction occurs when only one electron is involved, and it is more likely to generate organic lithium salt components.
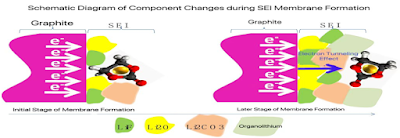
3.7. Question 3 - Composition of SEI
l During the initial stage of SEI film formation, the film-forming process can be divided into the following steps:
n Step ①: A large number of electrons are transferred from the current collector, through the conductive agent, to the surface of the graphite particles.
n Step ②: Under the action of an electric field, film-forming agents, solvated lithium ions, and other components diffuse to the surface of the graphite particles.
n Step ③: A large number of electrons on the surface of the graphite particles react with the film-forming agents and lithium ions to form the SEI film.
l A large concentration of electrons gathered on the surface of the graphite particles, a two-electron reaction occurs, leading to the formation of an SEI film dominated by inorganic components.
l During the later stage of SEI film formation,
n in addition to the above three steps,
n the process involves electrons penetrating the already formed, electronically insulating SEI film to reach the surface of the SEI film and react further with film-forming agents and lithium ions to generate additional SEI film.
l As the number of electrons that need to traverse the already formed SEI to reach the reaction site decreases, a one-electron reaction occurs, resulting in the formation of an SEI film dominated by organic components.
3.8. Key Control Factors for Formation Process
l Critical Factors Influencing SEI Film Composition:
n Electron Density
n Concentration of Reactive Substances
l Which process parameters affect electron density and reactant concentration? These parameters are the key points that must be strictly controlled in the formation process.
4. Canrd Brief Introduce
Canrd use high battery R&D technology(core members are from CATL) and strong Chinese supply chain to help many foreign companies with fast R&D. We provide lab materials, electrodes, custom dry cells, material evaluation, perfomance and test, coin/pouch/cylindrical cell equipment line, and other R&D services.
Email: contact@canrd.com Phone/Wechat/WhatsApp: +86 19867737979
Canrd Official Web Canrd Company Vedio Canrd Company profile
Website : www.canrud.com
5. Q & A
In this Q&A session, Dr. Ke answered all the questions raised by the participants.
Could this be love: "What's the relationship between electrode compaction and impedance?"
Dr. Ke: "Electrode compaction affects both electronic resistance and ionic resistance, which will certainly impact Rs (solution resistance), and it will also have some effect on Rct (charge transfer resistance)."
Crayon Little Pig: "If after assembling the battery, the EIS can't be measured, what could be the reason?"
Dr. Ke: "It could be an issue with the battery or the testing equipment. If the battery is fine, good testing equipment should be able to provide a smooth EIS curve."
Hei Hei: "If there is an overlap in the data, should we focus only on the non-overlapping parts, or can we still interpret the overlapping sections?"
Dr. Ke: "The overlapping parts can still be separated by peak fitting."
Li-ion anode: "Is there a direct correlation between the Nyquist plot and battery performance? I found that during cycling, the RSEI (SEI resistance) changes, but the charge-discharge capacity remains the same."
Dr. Ke: "Yes, there is a correlation, mainly with the rate capability. It's reasonable that RSEI changes while the capacity remains unchanged, as this only affects the charge-discharge voltage gap."
Li-ion anode: "Does this phenomenon also occur during constant current charging and discharging?"
Dr. Ke: "This is related to the formation current, and we will discuss this in detail in the next lecture."
West China University of Technology: "Dr. Ke, may I ask a question? In a lithium-ion battery, does charge transfer at the interface involve electron gain or loss? In the Rct you just showed, Li+ has a fixed valence state in a lithium-ion battery. For example, during discharge, it should be the transition metal gaining electrons, not Li+. Also, if it's about electron gain or loss, the diffusion in the solid phase wouldn't involve Li+ anymore."
Dr. Ke: "Indeed, diffusion in the solid phase isn't ionic diffusion but atomic diffusion. After Li+ penetrates the SEI, it exists in atomic form, not as Li+."
West China University of Technology: "So, during interlayer migration in LCO, it's lithium ions moving?"
Dr. Ke: "Yes, that's correct."















No comments:
Post a Comment