Today, let's introduce the detection indicators of the electrolyte.
HF, moisture, conductivity, density, and color are the five major control indicators in the industry. Moisture and HF are well known to everyone, and the general electrolyte moisture is controlled at 20PPM and HF at 50PPM.
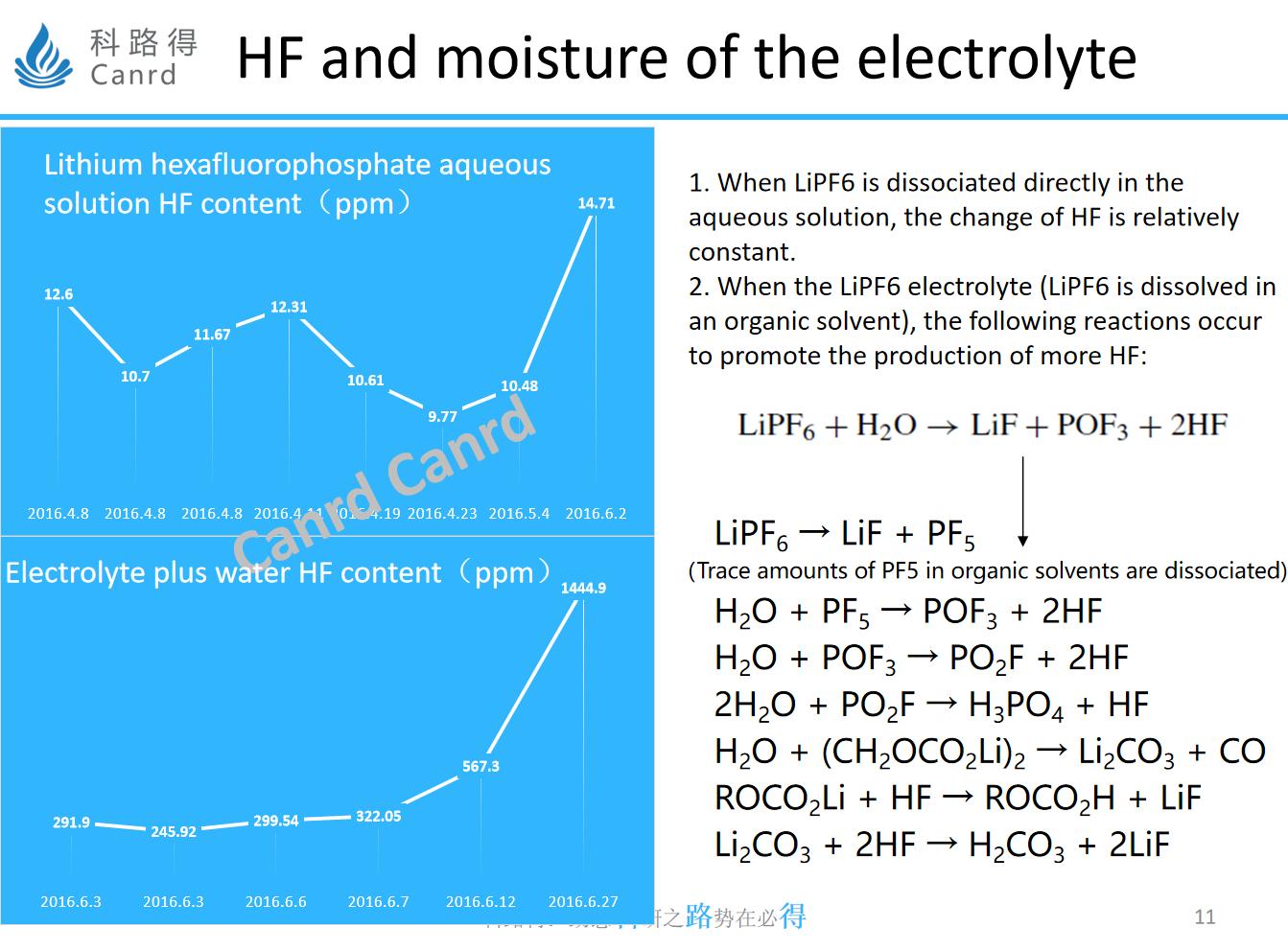
This is an experiment that we have done to explore the stability of lithium salts, the difference between lithium salts in an aqueous solution and an organic solvent. In fact, even if it is placed in water for 2 months, the change of HF is very small, but in organic solvents, it soars from 291 to 1444ppm in one month, and the main reason is that the dissociated lithium salt will have PF5, and PF5 will continue to trigger a series of subsequent reactions, resulting in the continuous generation of HF.

Karl Fischer moisture tester is generally used for moisture, and acid-base titration is used for HF.
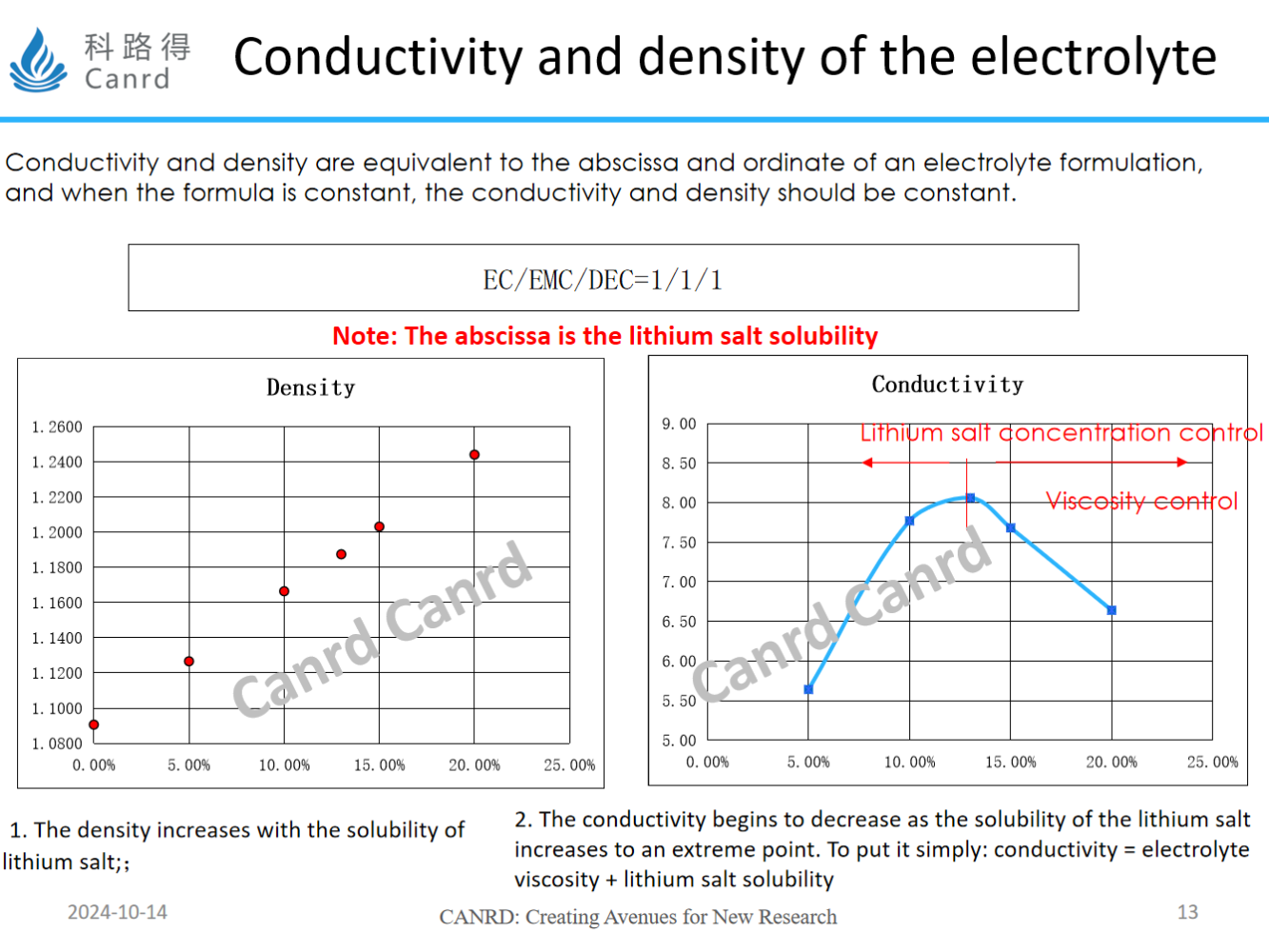
Regarding the conductivity and density of the electrolyte, the density increases as the concentration of lithium salts increases, but is it true that the more lithium salts are added, the higher the conductivity? As can be seen from the interaction between the two in the figure on the right, the conductivity is not only related to the concentration of lithium salt, but also related to viscosity, with the increase of lithium salt concentration, the viscosity will also increase, affecting the transmission of lithium ions, so the conductivity decreases after an extreme value. Of course, depending on the solvent system and temperature, this extreme point will also vary.
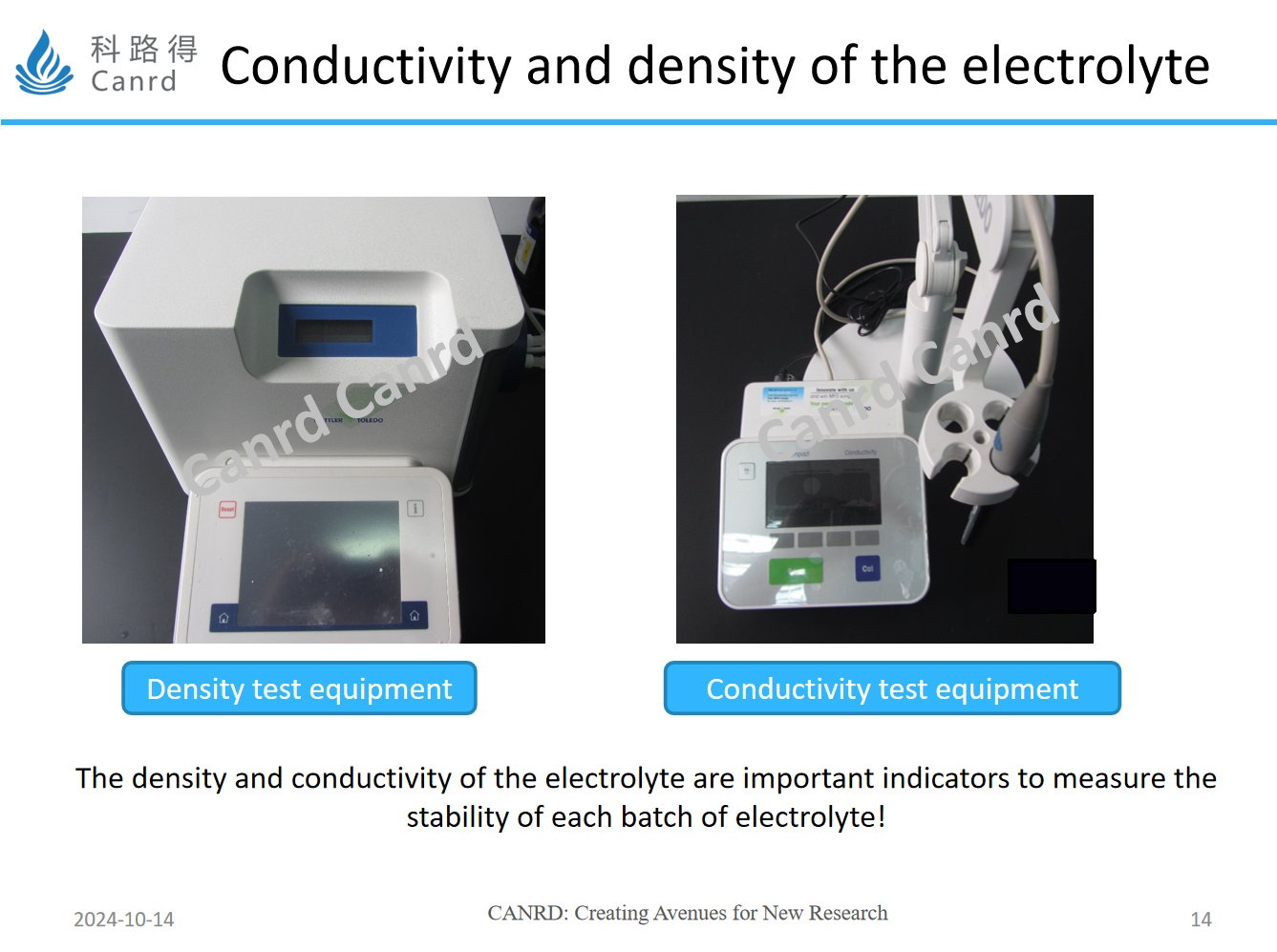
In addition, the conductivity and density of the electrolyte are tested by corresponding equipment, and each batch of Canrd’s electrolyte is strictly tested before shipment, so please feel free to use it.
Finally, the chromaticity of the electrolyte. With the development of new materials, systems, and demands, such as silicon anodes, 5V high voltage, high nickel, etc., we have found that the discoloration of the electrolyte has begun to increase.
Under normal circumstances, our industry recommends that the shelf life of the electrolyte is 3 months, not more than half a year, and some special additives and formulations also require low temperature storage. Generally, according to these requirements, there is basically no problem of discoloration, but in some special types of electrolytes, we find that the electrolyte will change color in a month, or even half a month in summer. We have made some summaries, and in the case of appealing the four types of high concentrations of EC, DMC, FEC, and lithium salts, the color development phenomenon is more obvious, so many times, in face of the application of some new systems, we will continue to find some new problems.
Does discoloration mean it can't be used? We have done a lot of experiments in the industry, when the chromaticity is controlled within 200 chromaticity, in fact, there is no effect on the capacity, first effect, internal resistance, circulation, rate, and storage.
Finally, I will discuss the development direction of electrolyte. We categorize it into the above four directions, the first is a wide temperature application, that is, it is necessary to meet both low temperature and high temperature; The second is a high-safety electrolyte, which I think will be gels and finally solid-state electrolytes in the future. The third is high voltage, some students mentioned earlier that there is no problem with 5V under CV, does it mean that it can be applied with high voltage, which often ignores the difference in materials, surface activity and catalytic performance of materials; The last is the development of functional type, according to the customer's application scenarios, such as the military needs of extremely low temperatures, the temperature requirement is -60 °C; Special high temperature applications 85°C; The need for high rate, such as the instantaneous discharge of the igniter above 100C; In the future, the long service life of automobiles, which can be cycled 5,000 times, etc., will have different requirements for electrolyte.