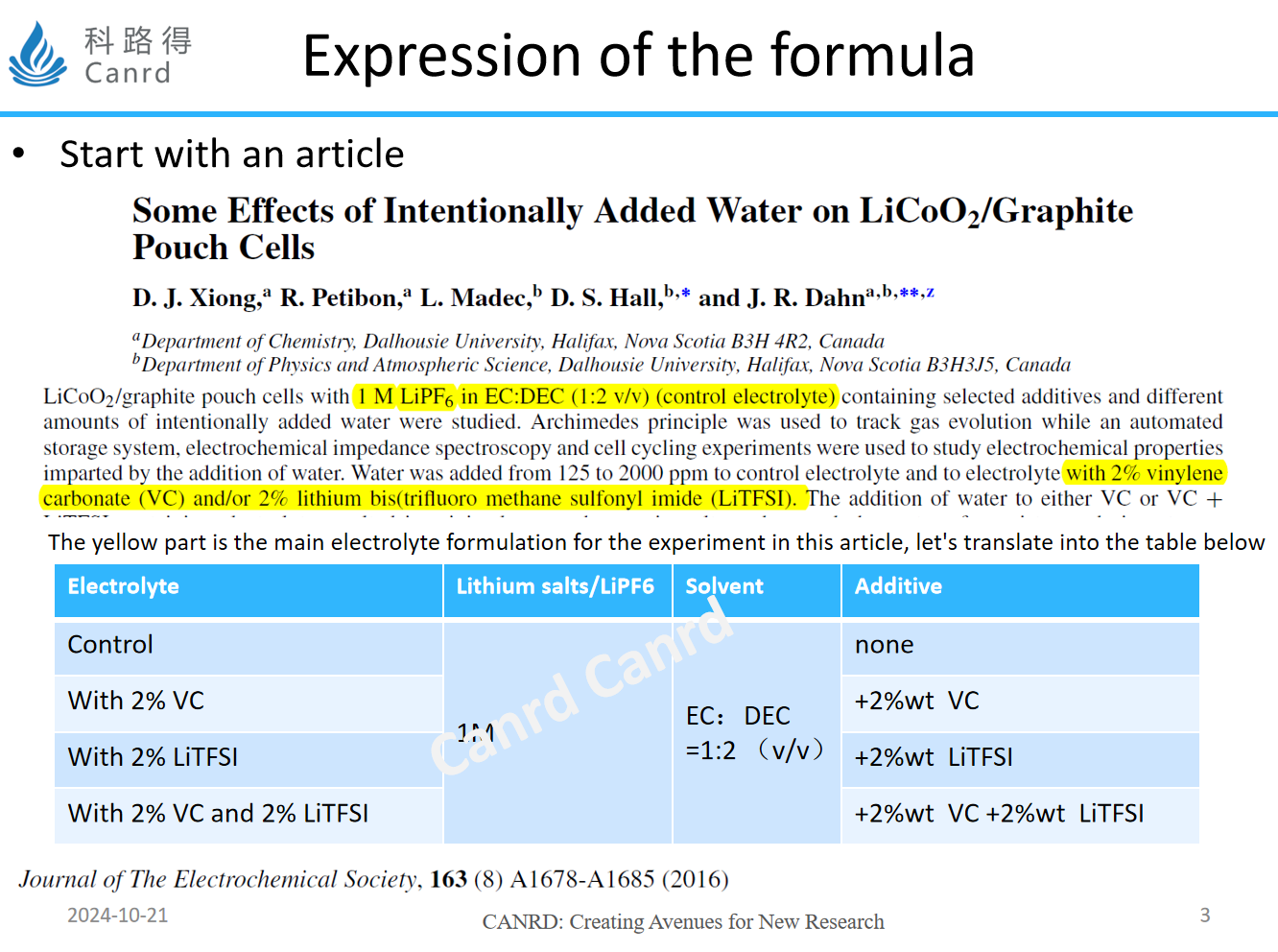
First of all, we want to introduce the expression of the formulation, which is an article by Jeff Dahn that contains the specific requirements of the formulation, including the proportion of solvents and the amount of additives to be added. Here we note that the lithium salt is 1mol/L, the solvent is the volume ratio, and then addes additives in different proportions.
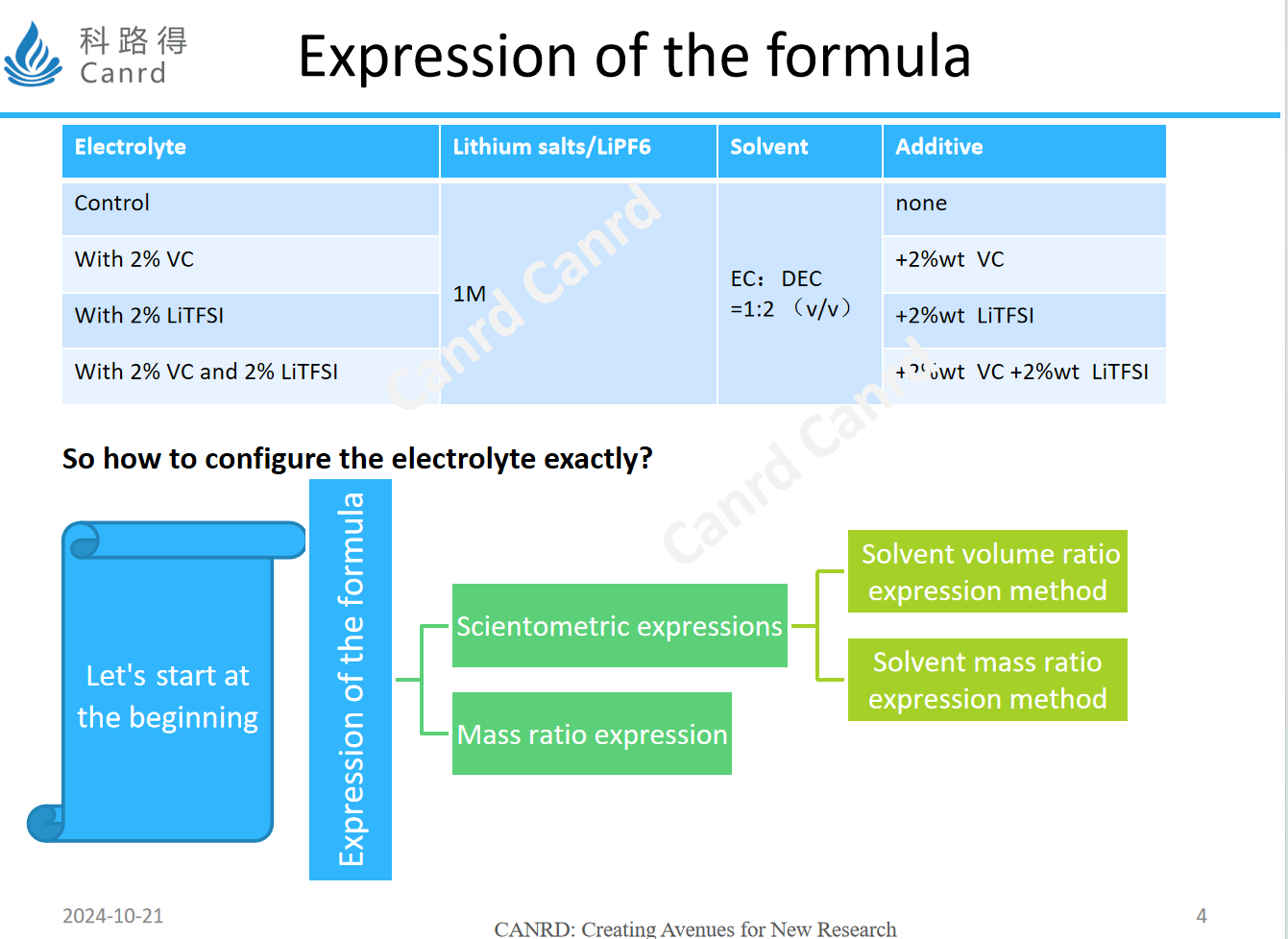
General formula expressions are divided into scientometrics and mass ratio expressions, while scientometrics have two types: volume ratio and mass ratio, let's look at specific examples later.
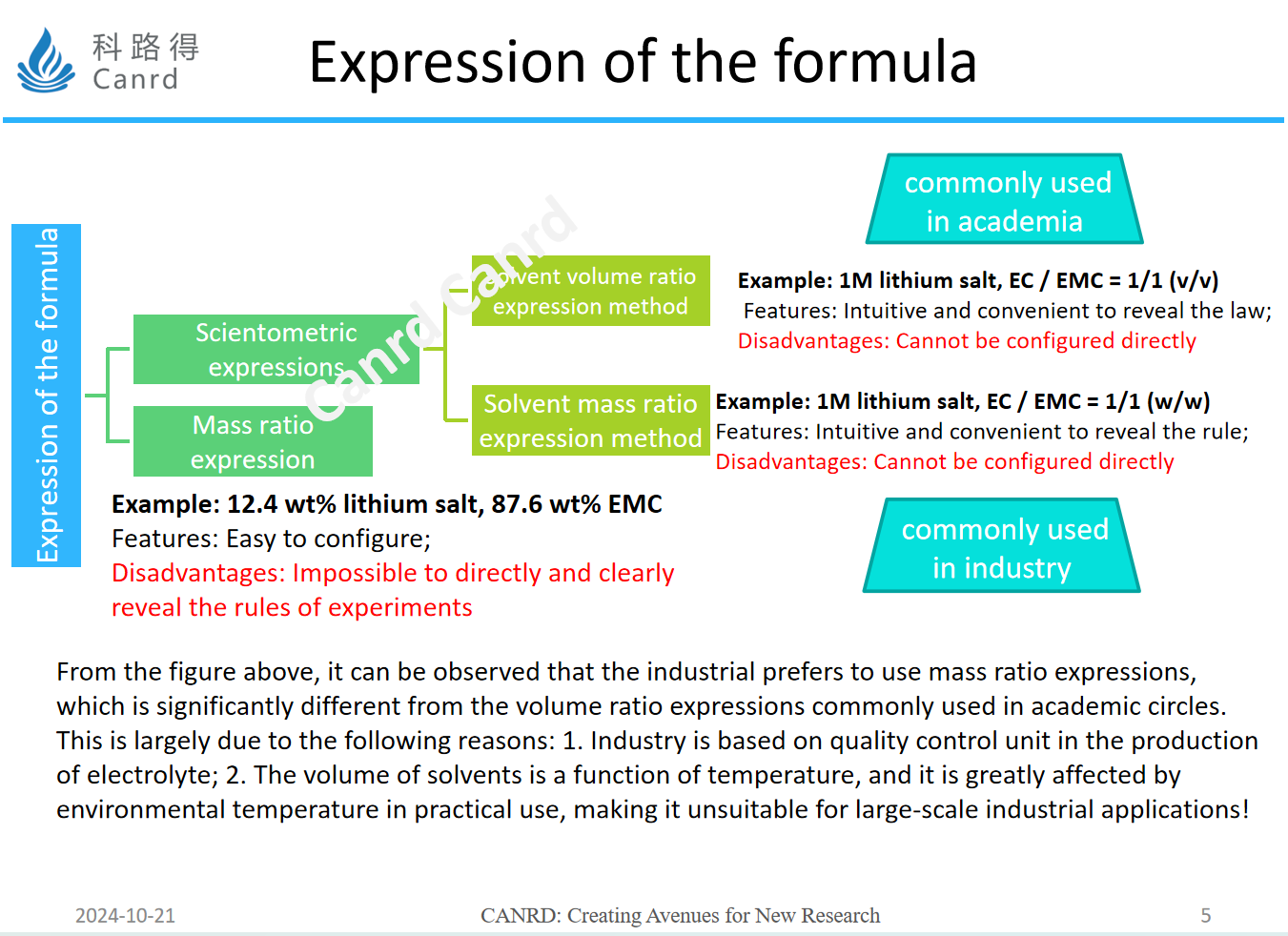
For example, the mass ratio of the first type is more intuitive, which tells you what is the mass ratio of lithium salts in an electrolyte, and what is the mass ratio of solvents and additives. It's very easy to configure, but there's no way to tell us exactly what the proportions are, so let's look at scientometrics. The advantage of this method is that it is intuitive to reveal the rule, what is the proportion of solvent, and how many mol of lithium salt is. The volume ratio is the volume we have learned since junior high school, and it is configured with volumetric flasks and graduated cylinders, but in the industrial, this method is not suitable, because the environment is certainly not like a laboratory, and the volume is related to the change of temperature, only the mass can be stably weighed, so the industry uses the mass ratio.
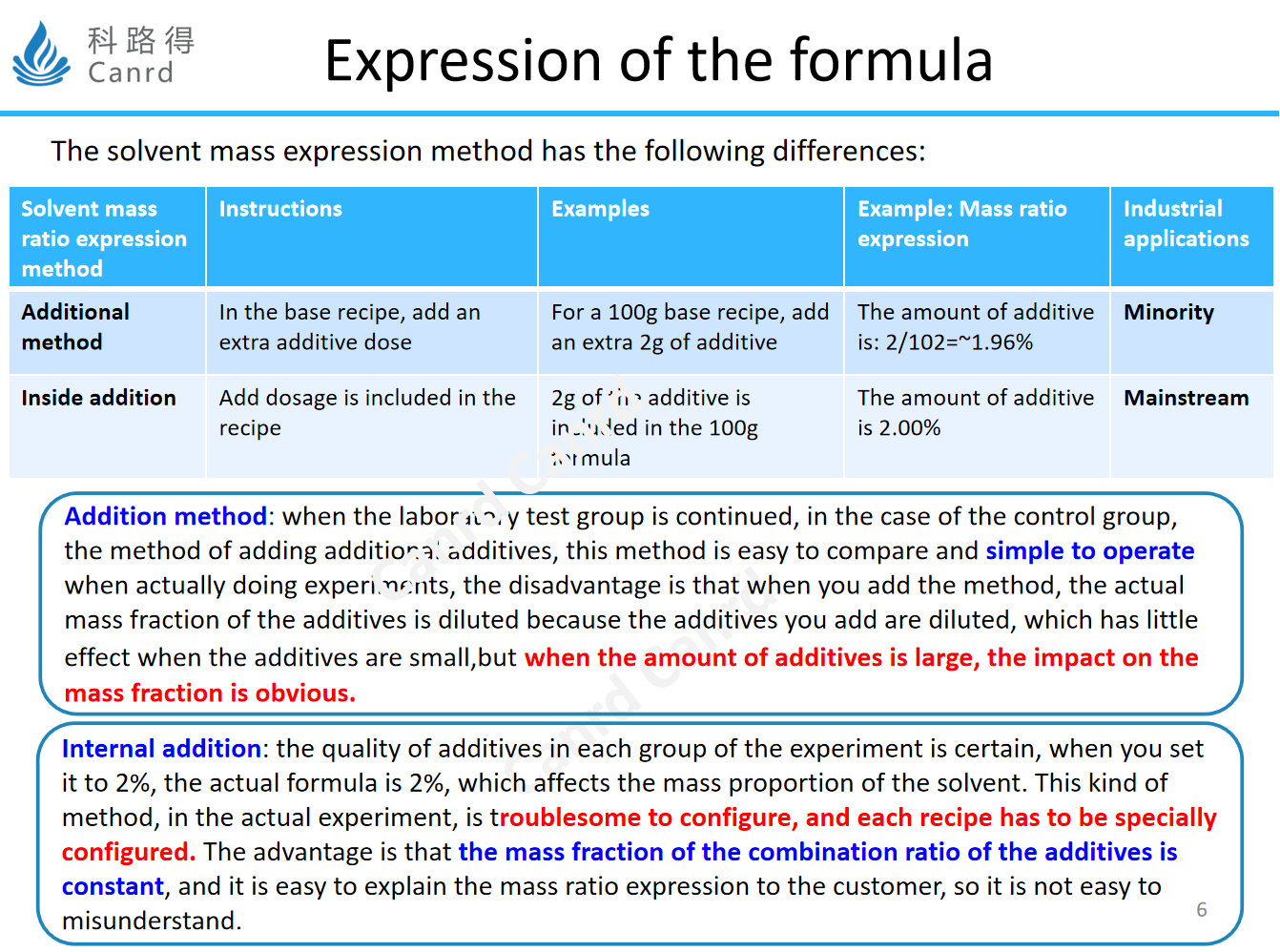
When using the mass expression method, we often have two ways for additives, namely external addition and internal addition, we can look at the table. The mainstream addition method is that the overall mass percentage is to include the added additives, so that the ratio of additives is fixed, but each formula needs to be configured separately and calculated separately, which is relatively troublesome.
Therefore, there is also a relatively trouble-free, that is, the basic formula stipulates, the amount of additional addition is directly added on the basis of the previous one, so that a variety of electrolytes can be quickly configured, if the amount of additives is not much, the difference is not obvious, such as 2% and 1.96% in the above table, but if the amount of additives is more, or when there are many types, it is to dilute the basic formula, so that the proportion of additives will change. The mainstream of the enterprise is still according to the internal addition method, because the types of electrolyte additives that are truly commercialized are generally 4~5 kinds, or even more.
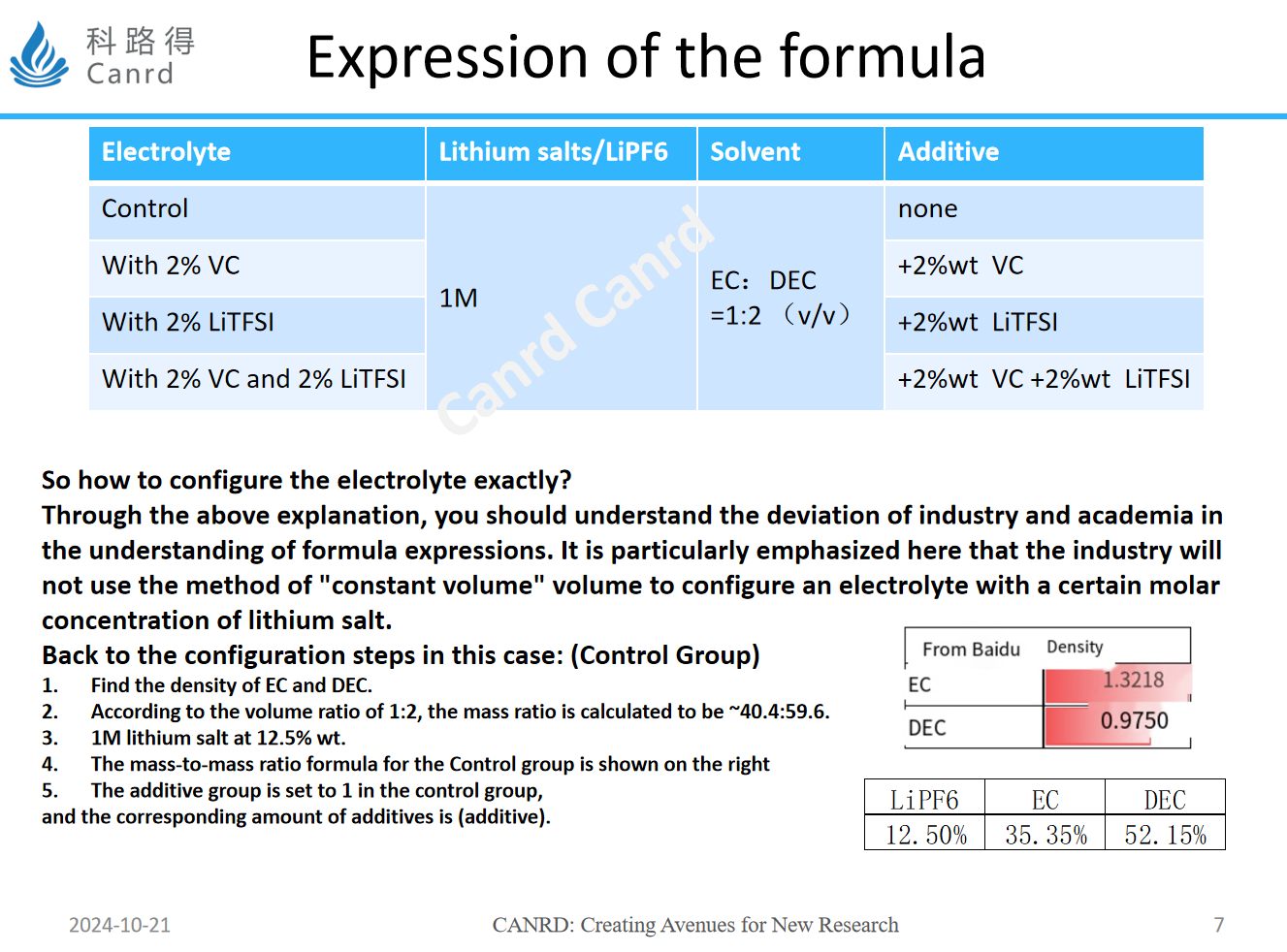
Finally, we can see the electrolyte configuration method in the previous article. Because use the volume ratio method, the density is converted into mass, and then the additive method is added by the external method, which is more commonly used in colleges and universities.
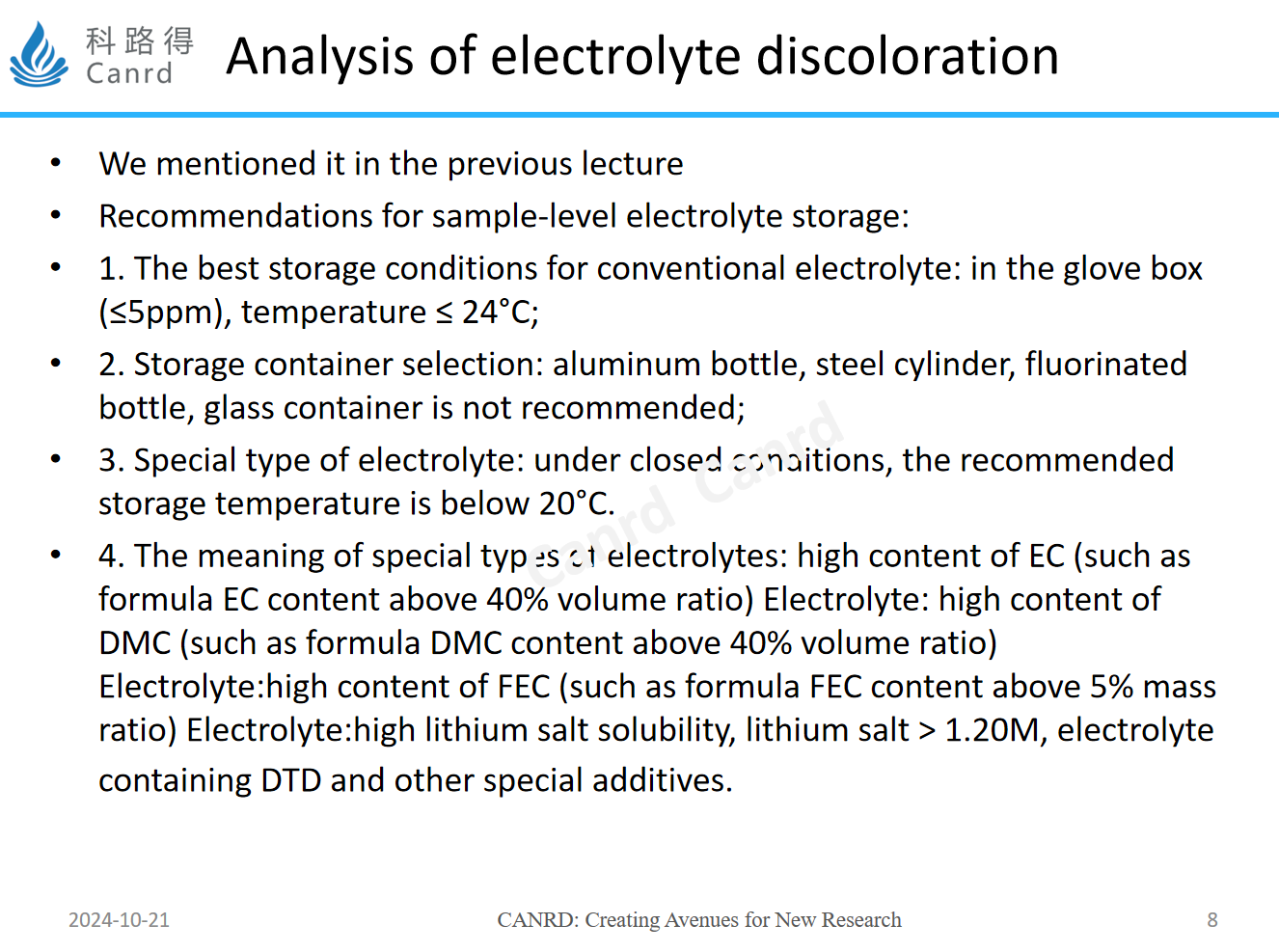
This content should be of interest to everyone. For sample-level electrolytes, we have some storage recommendations. In addition, some special types of electrolytes are also deliberately proposed here, not to say that the moisture or HF in the electrolyte configuration process, or the environment is not well controlled, but that the stability of this special type of electrolyte is poor, so it is easier to change color than ordinary electrolytes under the same storage conditions.
What factors affect the discoloration of the electrolyte? There are three main factors – lithium salts, the type and content of additives, and the environment in which they are stored.
The discoloration of electrolytes is often complex, and the related factors have a relevant influence, so it is often difficult to predict. We'll take a look at some of the identified influencing factors using a simple experiment of optimization.
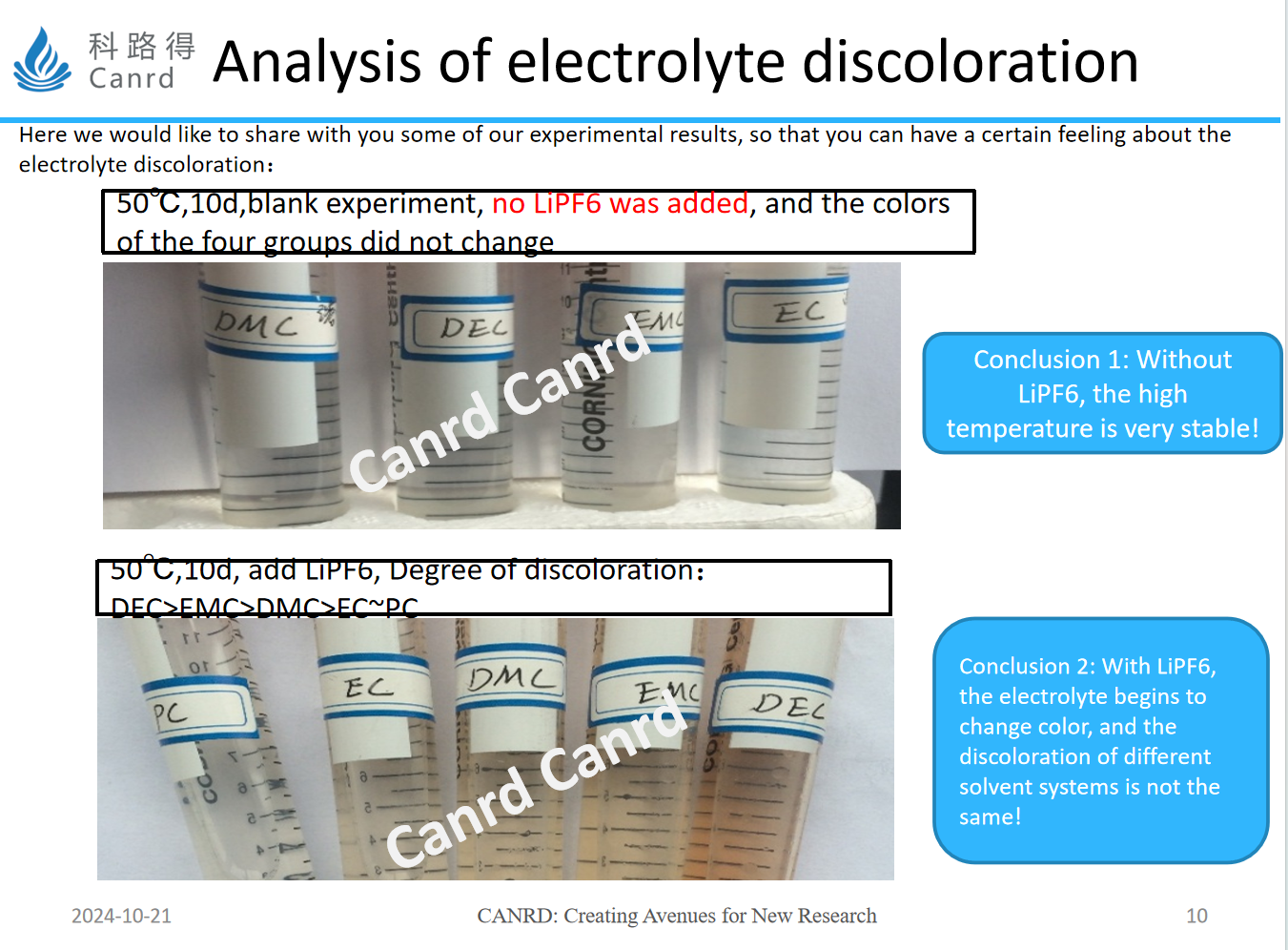
Two experiments were done here, the first was without the addition of lithium salts, and the contrast of different solvents, after 10 days of storage at 50 °C, did not change color. Next, we added lithium salts, and after 10 days of storage, we found that the color began to change, DEC, EMC, DMC all changed color, and DEC was darker than the color.
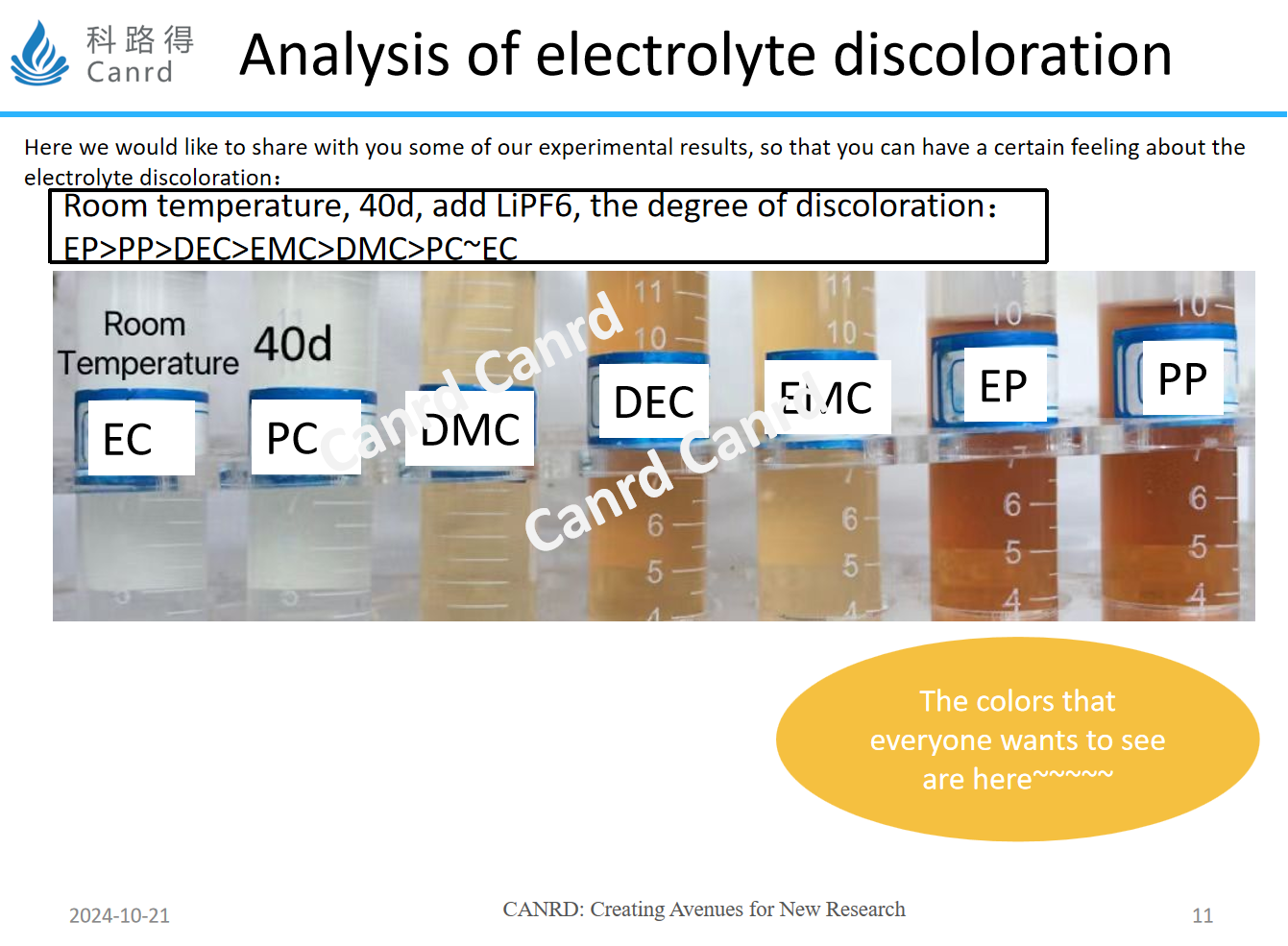
In this experiment, we changed the conditions to room temperature storage, extended the time, and added carboxylate EP, PP. It turned out that the discoloration of carboxylate esters was the most severe, followed by the aforementioned DEC.
So the summary here is that lithium salts are a major factor affecting discoloration, compared with different solvents, carboxylate esters are the most susceptible to discoloration, and among the commonly used solvents, DEC discoloration is the most serious.
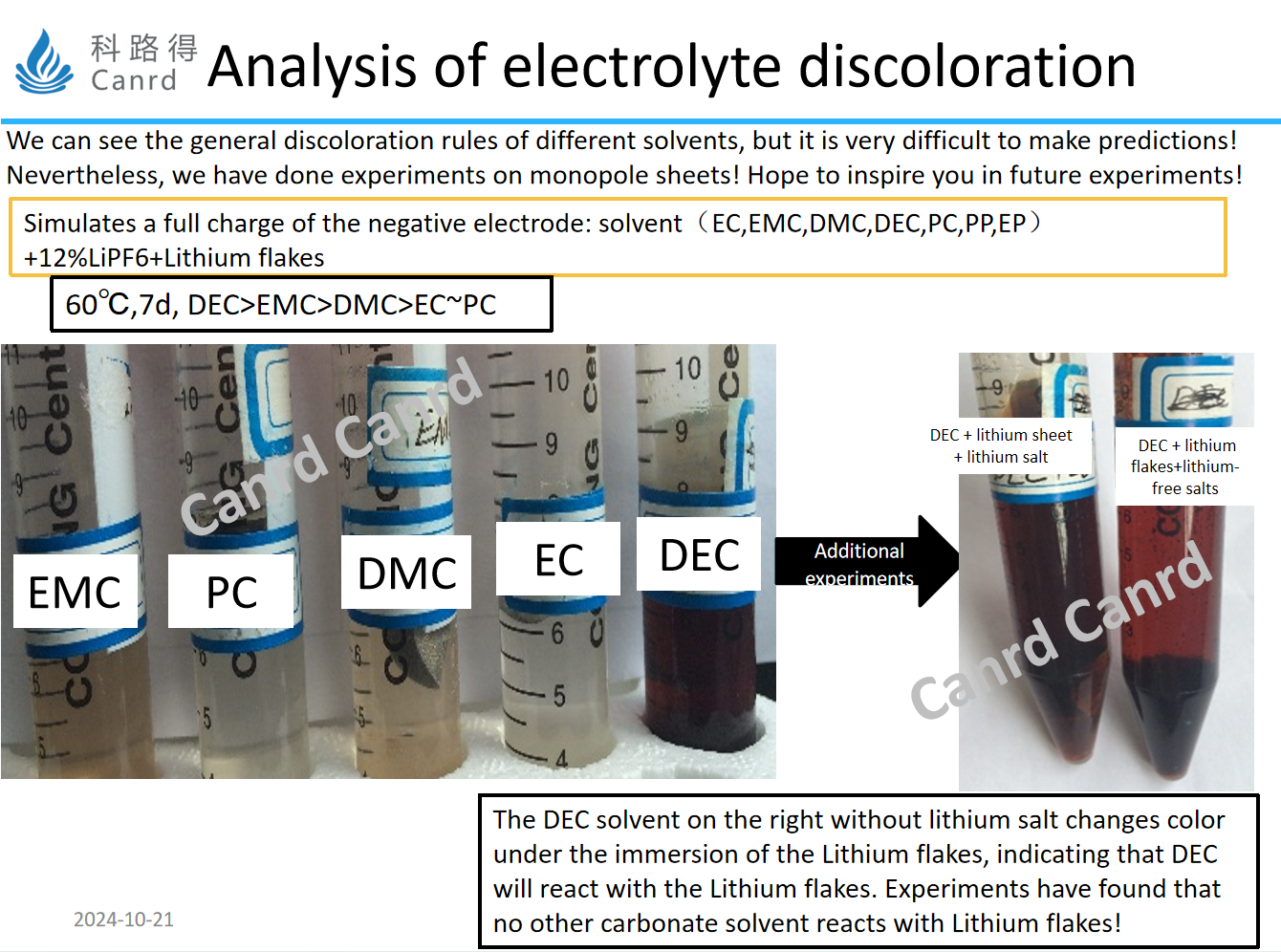
Next, we simulate the situation in the whole battery, in addition to the solvent and lithium salt, and use lithium flakes to replace the fully charged anode electrode. After seven days of storage at 60°C, the DEC discoloration was still the most severe, and it was almost black. Then we also did another experiment, looking at the picture on the right, we compared the effect of lithium salt on the discoloration of DEC+ lithium tablets, and found that even without adding lithium salts, DEC+ lithium tablets also showed discoloration.
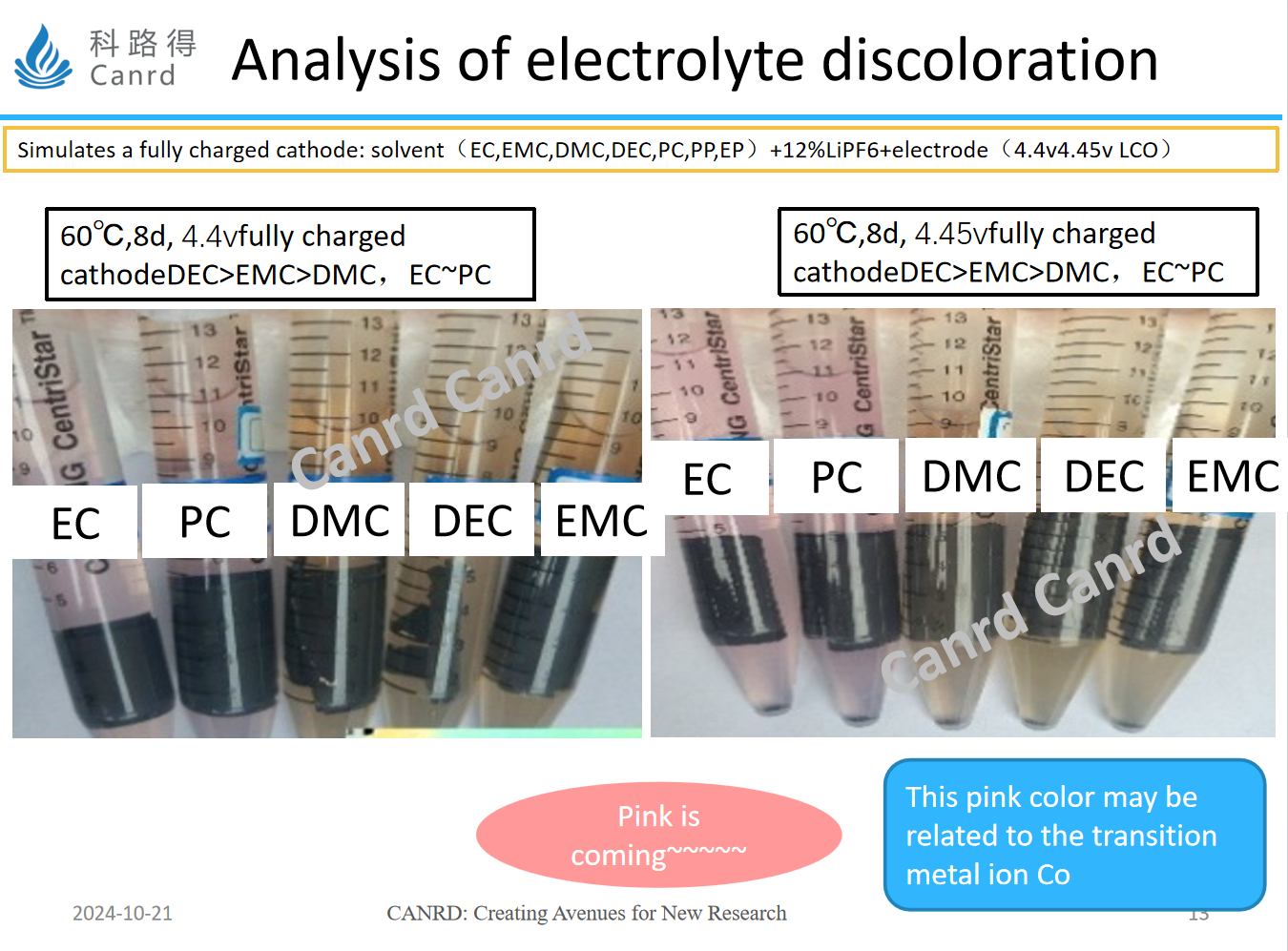
The same solvent + lithium salt + cathode electrode full filling piece.
The left side is 4.4V, the right side is 4.45V, and the phenomena on both sides are close. However, EC and PC have a new pink color, and we speculate that this is related to the transition metal ion Co (lithium cobalt oxide cathode). That is to say, for different electrodes, different solvent discoloration reactions are different.
Therefore, from the above small experiments, we can see that solvents, lithium salts and electrodes are all factors that affect discoloration. Of course, different temperatures and different storage times also have a significant impact on discoloration, so we will not list them one by one here. In our actual electrolyte configuration, there is more than one type of solvent and additive, and the amount of additives is also different, so the impact of discoloration is often more complex, but everyone agrees that when the electrolyte is stored at a high temperature, it is easier to change color after being placed for a long time.