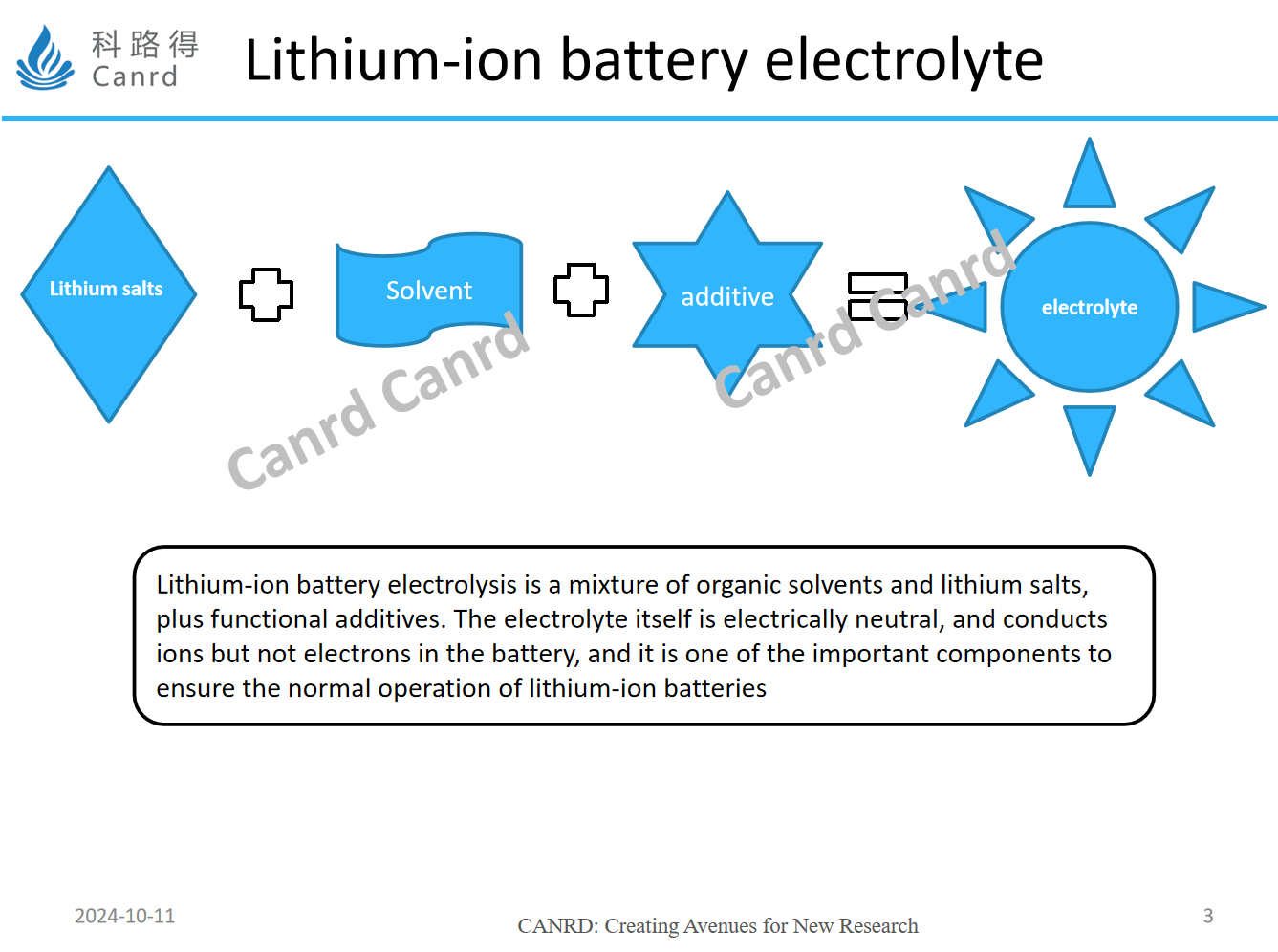
First of all, we will introduced the composition of lithium-ion battery electrolyte, which is generally divided into lithium salt + solvent + additives. Although the amount of additives is not much, it often has a great impact on performance, and is often compared to monosodium glutamate in electrolyte. Lithium salts are dissociated in organic solvents to form lithium ions and anions, resulting in ion conduction. From this point of view, the solvent must have a good dissociation ability, so that the lithium salt can be effectively dissociated. In addition, the solvent needs to have a low viscosity to ensure that the lithium ions are less resistant to transport in the electrolyte.
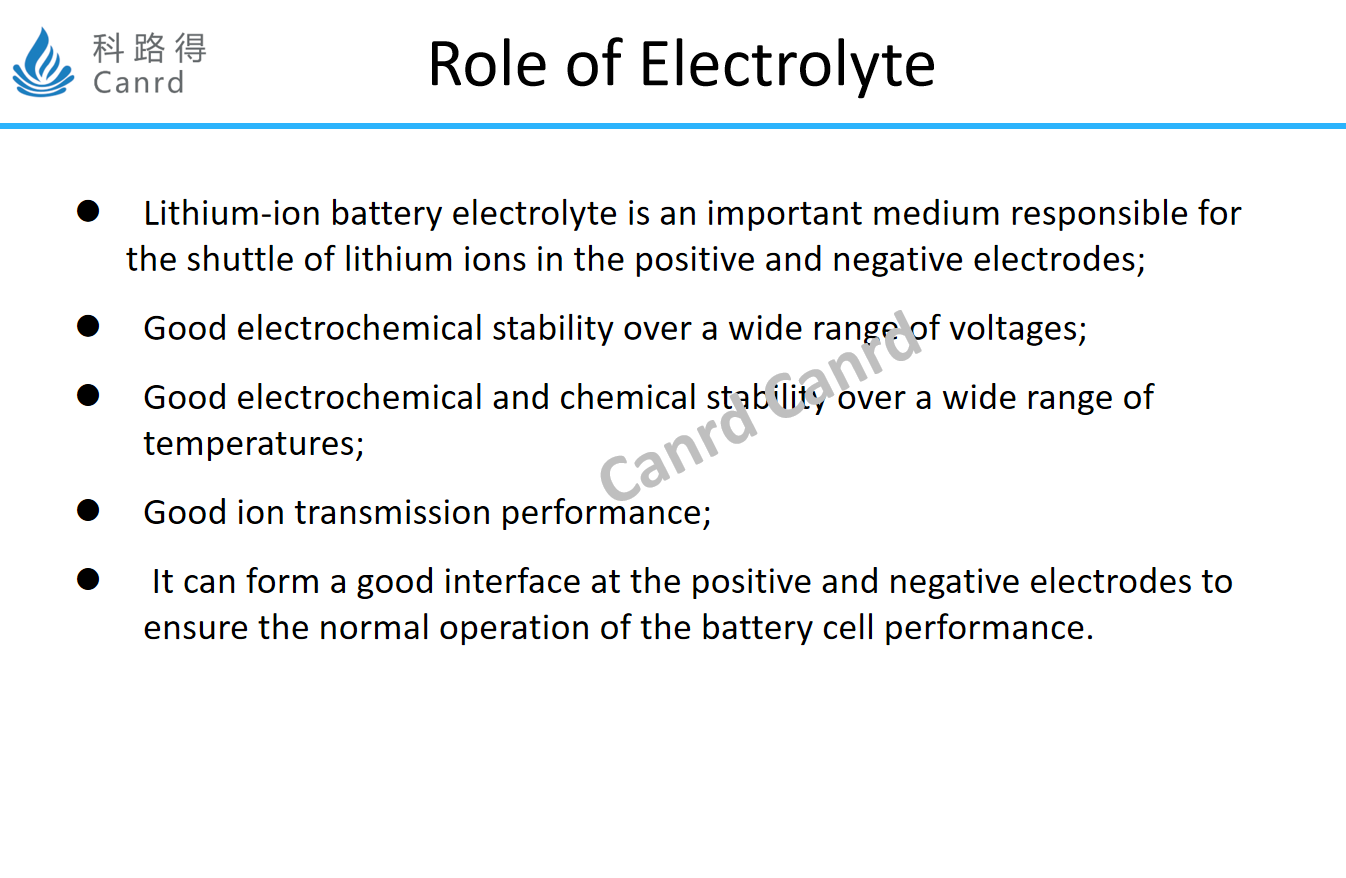
What are the functions of electrolyte? To put it simply, the ion conduction is good, the electrochemical stability is good, and can form an effective and stable interface, which includes both the well-known SEI and the interface formed with the cathode. Next, let's take a look at the common components of electrolytes.
Let's start with a few common solvents. This includes cyclic esters, such as EC and PC, followed by chain esters, including, DMC, EMC, DEC.
As mentioned earlier, EC and PC play a role in dissociating lithium salts because of their high dielectric constant; Chain esters have a low viscosity and are suitable for ion movement, so most electrolytes are mixed by combining the advantages of both.
In addition, EC has a better film-forming effect than PC, so in general, EC is the main electrolyte for long cycles, and PC controls the amount of addition. Different chain esters have different viscosity and boiling point, some high temperature requirements will consider DMC, while high temperature requirements will consider DEC. From our previous experience, the electrochemical stability of DMC is poor, and it is recommended not to be used in some high-voltage, high-nickel systems.
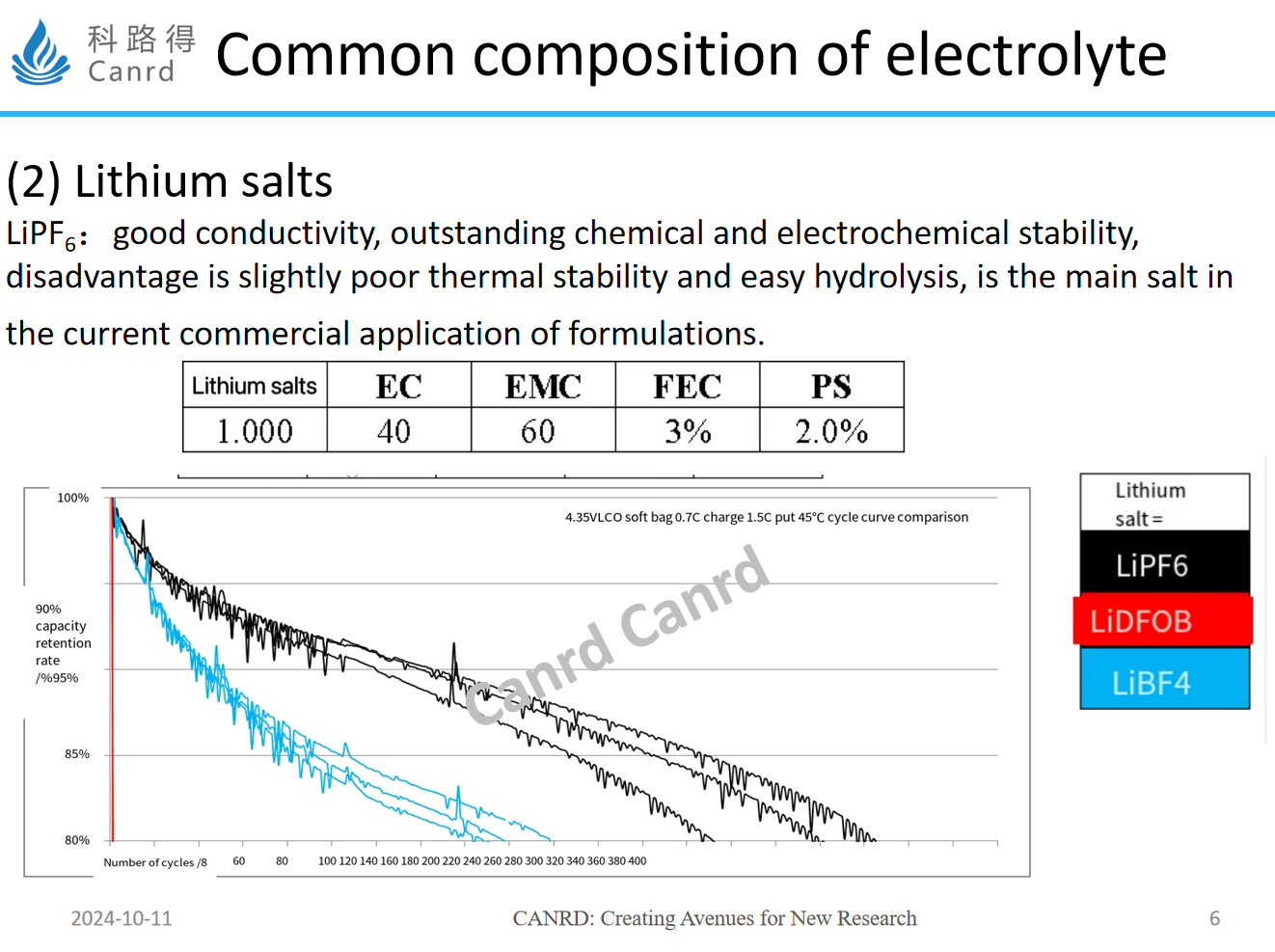
The second is lithium salt, here is just an example of the most commonly used three lithium salts, at present, lithium hexafluorophosphate because of its high conductivity and electrochemical stability, has become the most widely used electrolyte. As we can see from the cycling performance of pouch battery, the performance of the other two lithium salts is relatively poor. Particularly ODFB is attenuated after only a few weeks of circulation, mainly due to the poor film-forming of lithium salts and the abnormally large internal resistance. Therefore, at present, these two lithium salts are often used as additives in electrolytes, and LiBF4 is improved for low temperature and is applied to low temperature electrolyte systems.
Let's take a look at some of the common additives. VC is the most widely used type of film-forming additive, with good film-forming performance and significant improvement of circulation, but the impedance of film-forming is relatively large. The FEC developed later has a high reduction potential and a small impedance for film formation, because it has also been widely used, especially some new anodes, such as silicon anodes, both of which must be added. However, FEC has a relatively big problem, that is, high-temperature storage of gas production. Therefore, the performance is often balanced in the electrolyte because of some other additives.
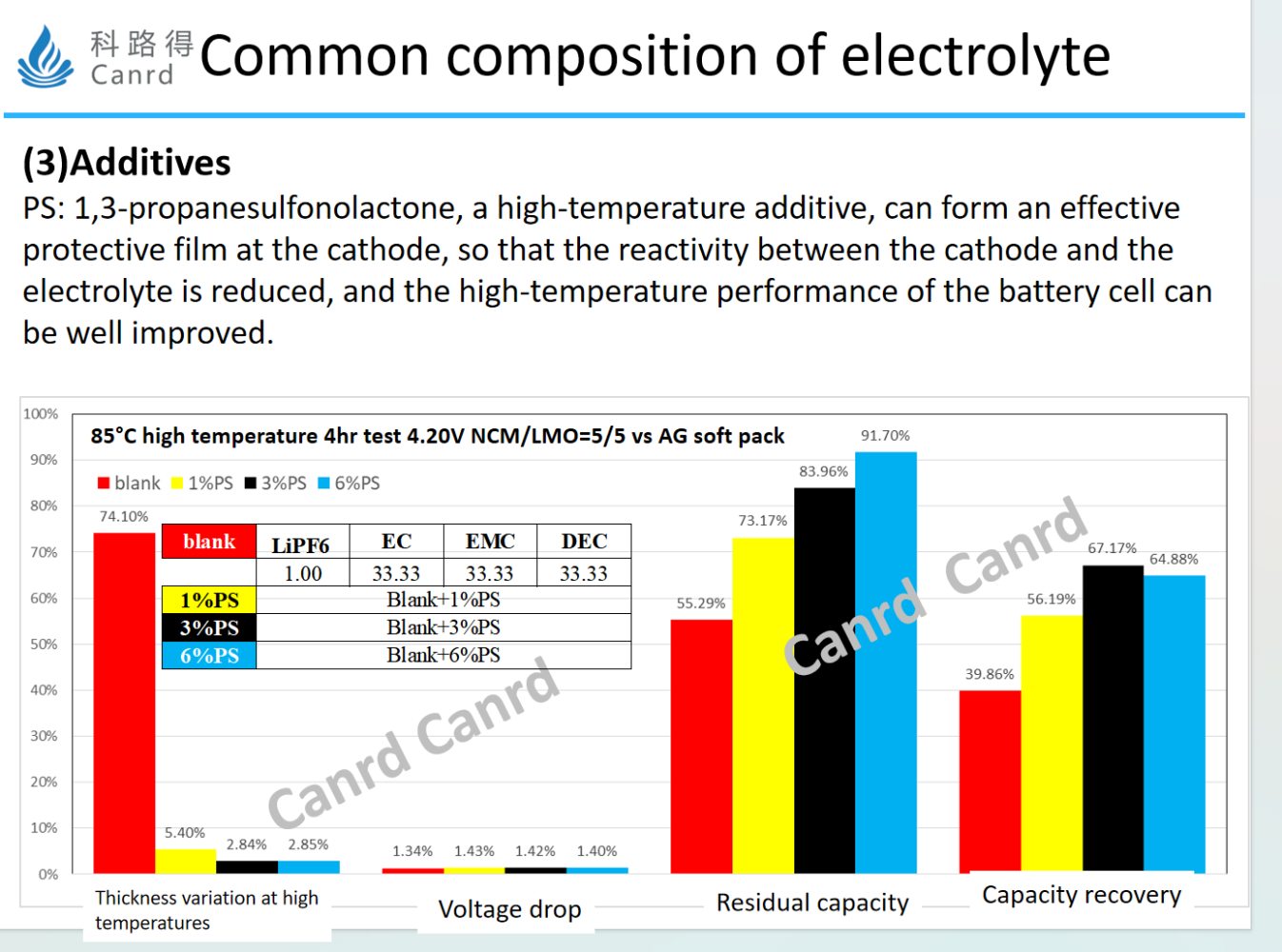
PS, an effective cathode film-forming additive, as can be seen from the storage data below, with the increase of PS content, the high temperature performance is effectively improved.
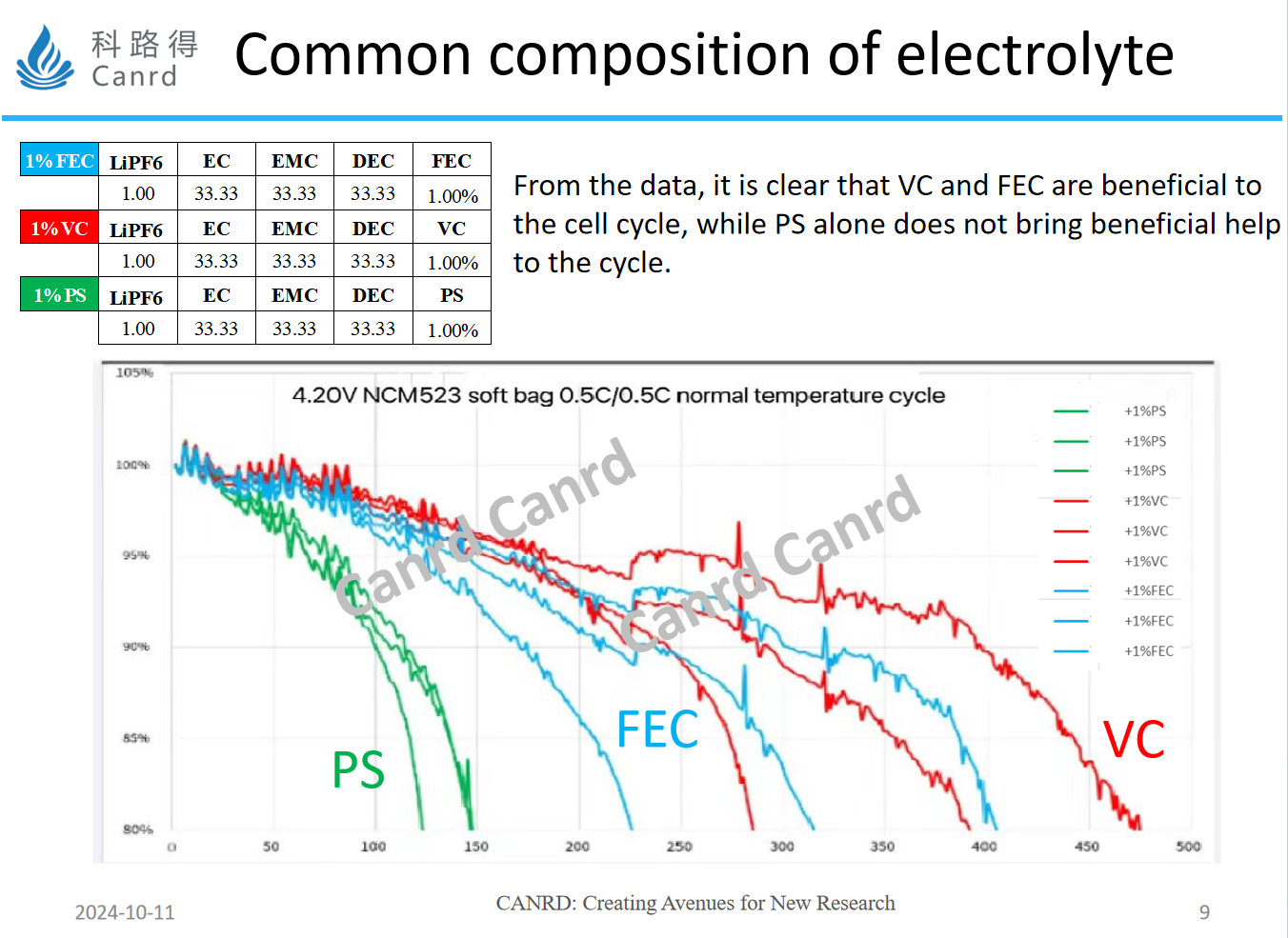
We compared the effects of the three additives on cycling in the whole cell, and from a cycling point of view, while PS can effective improving high-temperature performance, cycling had no advantage over the other two types of additives. Therefore, the actual commercialized electrolyte does not have only one or two additives, but three or more according to the need, all of which are to balance the performance of the whole battery cycle, high temperature, low temperature and so on. This is a big difference between universities and the industry, colleges and universities often pay attention to the certain performance, while the industry from the perspective of the product, need to ensure that the product can play a normal performance at different temperatures and different application requirements.
Here are just some examples of common lithium salts, solvents and additives, such as the carboxylate solvent, which is also very widely used in recent years, because with the increasing energy density of mobile phone batteries, the compaction density of anode graphite becomes larger, and the wettability becomes a major issue. Carboxylic esters have good wettability and are therefore widely used.