Today, we will mainly introduce the cathode materials.

This is the outline of today's introduction, first of all, a brief introduction to the basic principles of lithium-ion batteries.
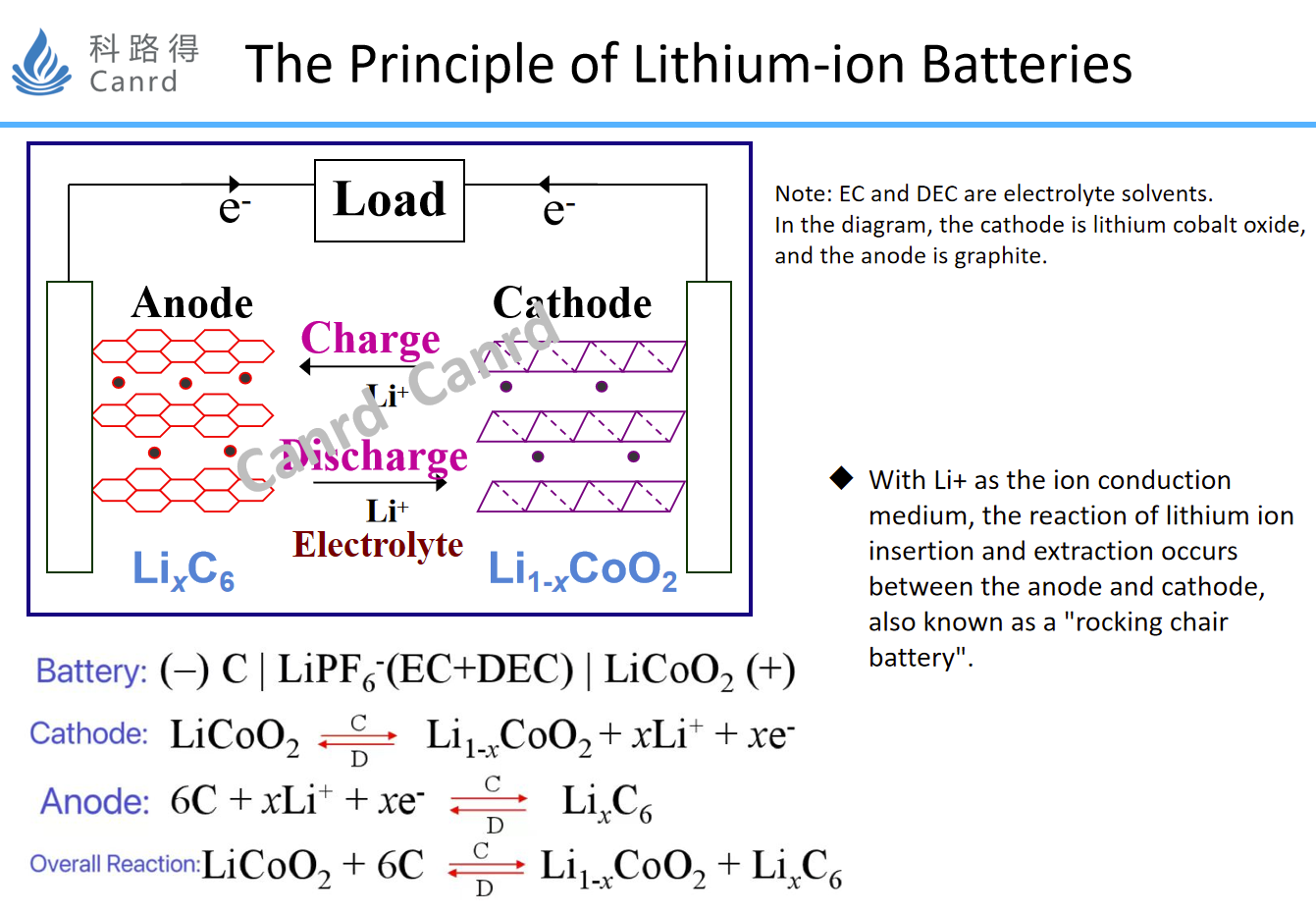
Lithium-ion batteries, also known as rocking chair batteries, because lithium ions are embedded and detached between the cathode and anode electrodes during charging and discharging. The cathode and anode electrodes have a stable layered structure, so the stability of lithium-ion intercalation and detachment is high. In 1990, Sony launched the first commercial lithium-ion battery, which replaced the previous metal lithium anode because of the use of stable carbon materials as the anode, so that lithium-ion batteries could be quickly popularized and commercialized.
What materials are the general lithium-ion batteries composed of?
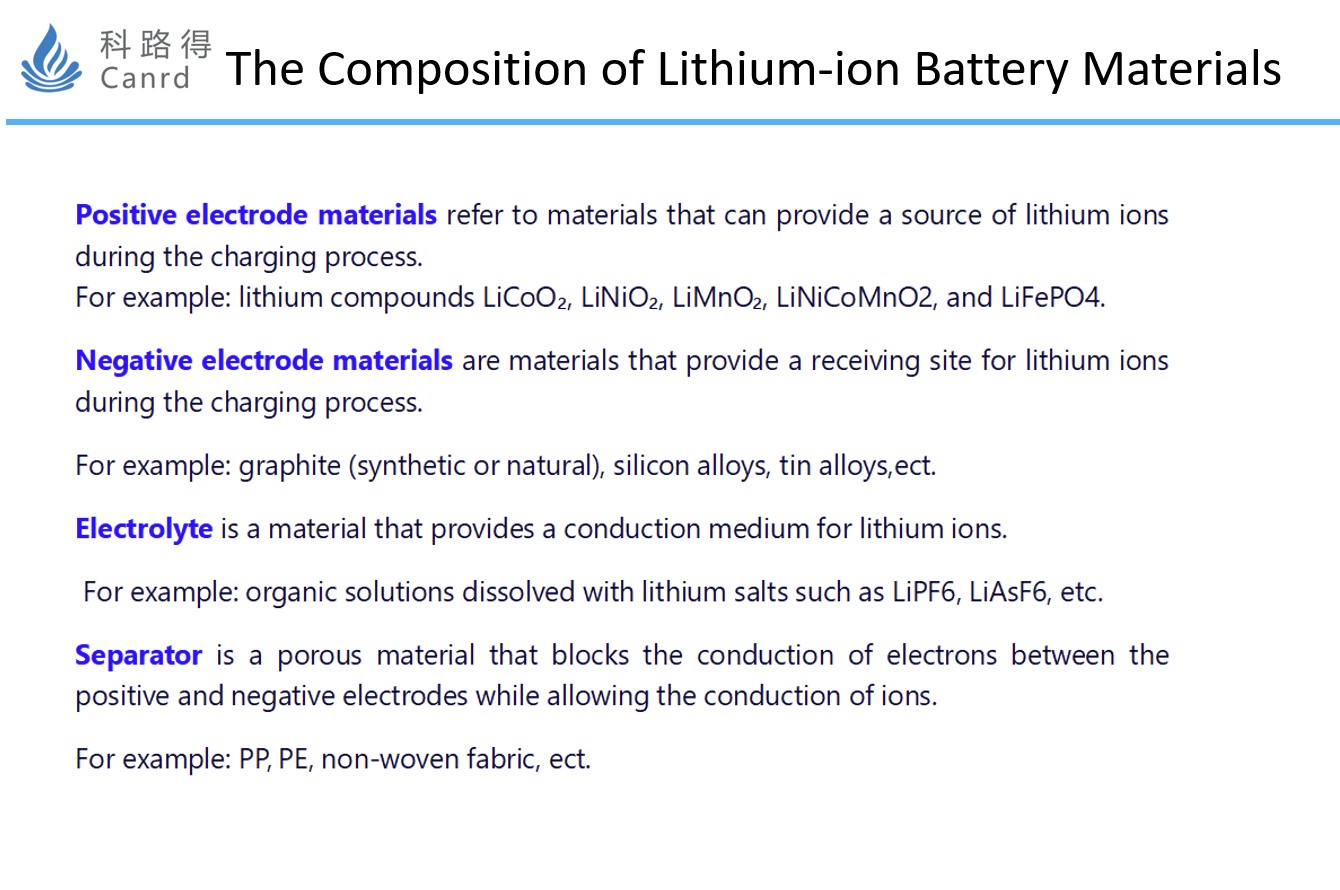
Cathode and anode electrodes, electrolytes and separators are the four main materials. Their roles are relatively easy to understand.

This is followed by other materials such as current collectors, packaging shells, conductive agents, adhesives, and electrolyte additives. Today mainly introduces cathode materials.
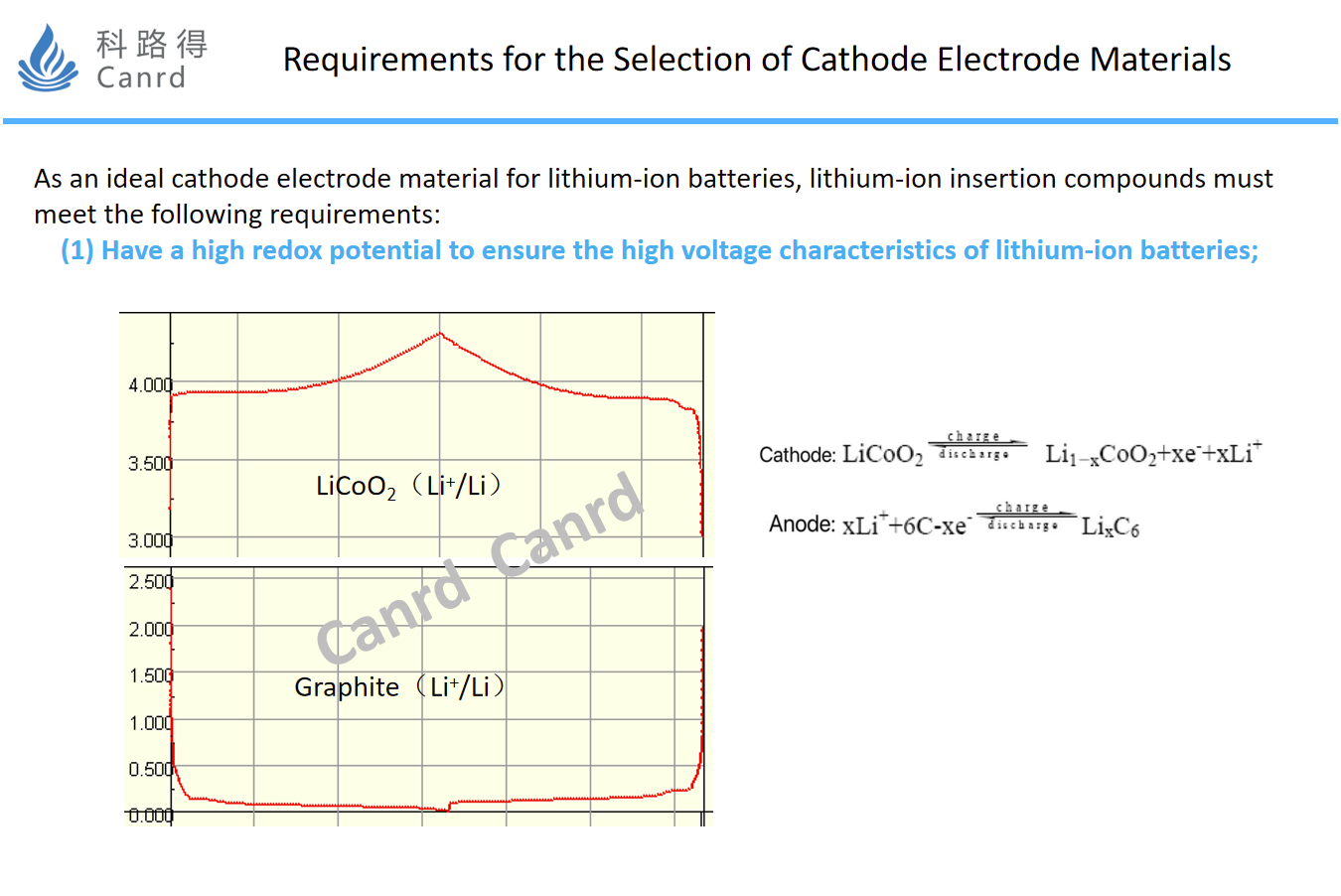
How to select the cathode material, the first point is to have a high redox potential, so that the assembled battery has high voltage characteristics and provides higher energy.

Secondly, more lithium ions need to be allowed to intervene and exit, i.e. the material has a higher capacity.

Other relevant requirements are as follows, including good structural stability, high electronic and ionic conductivity, good chemical stability to electrolytes, as well as low cost and good processing performance.
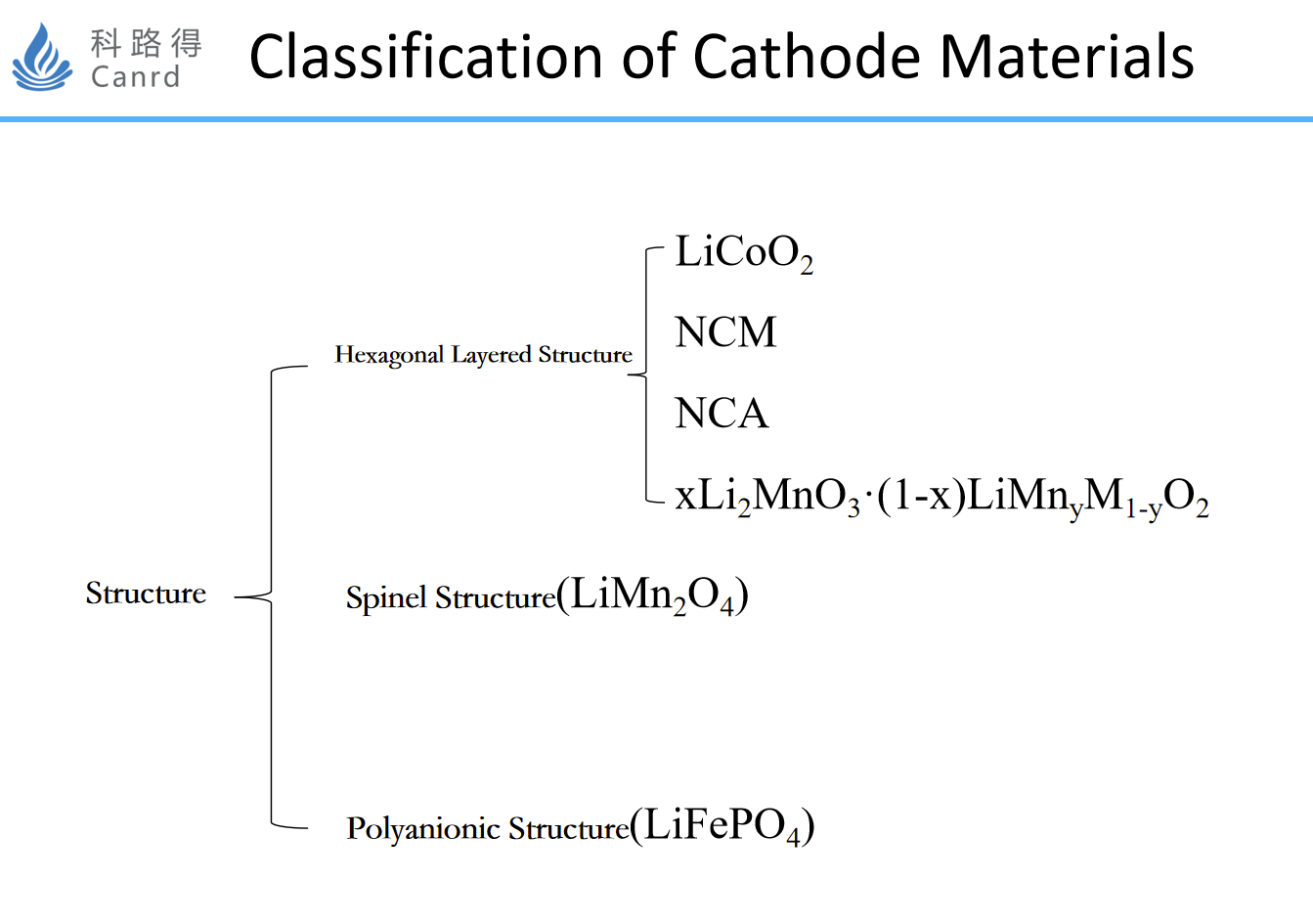
At present, there are three main classifications, layered, spinel, polyanionic (olivine type). Each structure has different characteristics and corresponding application directions.
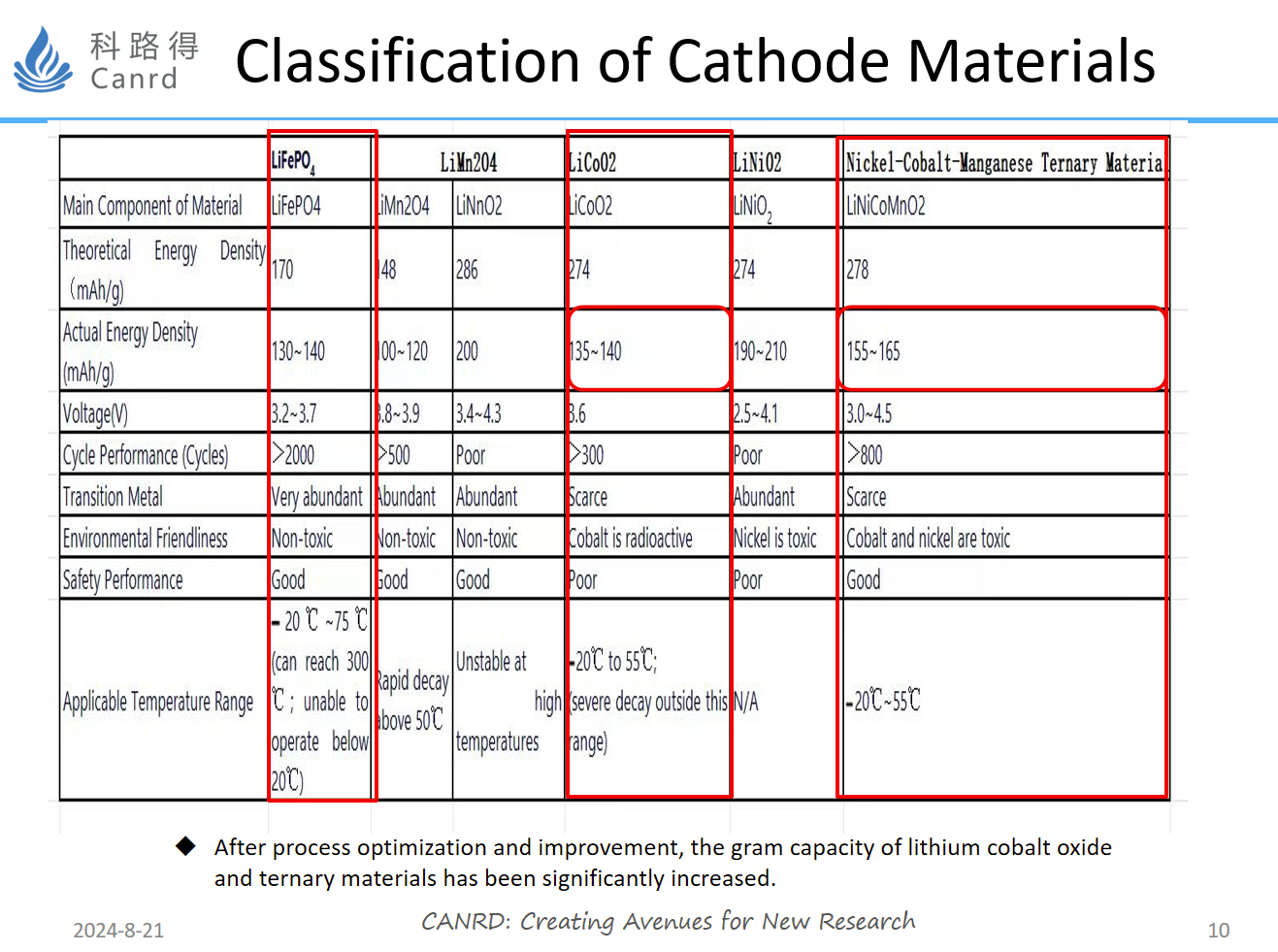
This diagram makes a classification and summary of commonly used materials. The most commonly used cathode materials include lithium iron phosphate, lithium cobalt oxide, and NCM. Lithium iron phosphate has the best structural stability, good cycle and safety, and the main disadvantages are low capacity, compaction, platform, and low energy density. Lithium cobalt oxide has the advantages of high voltage and high compaction, and the charging voltage is also increased through surface coating, and the mature has achieved 4.45v, and the gram capacity is also very high>180mAh/g. The disadvantage is that it is less thermally stable and safe, so it is suitable for consumer electronics, such as mobile phones. NCM materials are currently very hot materials, with the characteristics of high capacity and good circulation, and are mainly used in power batteries.
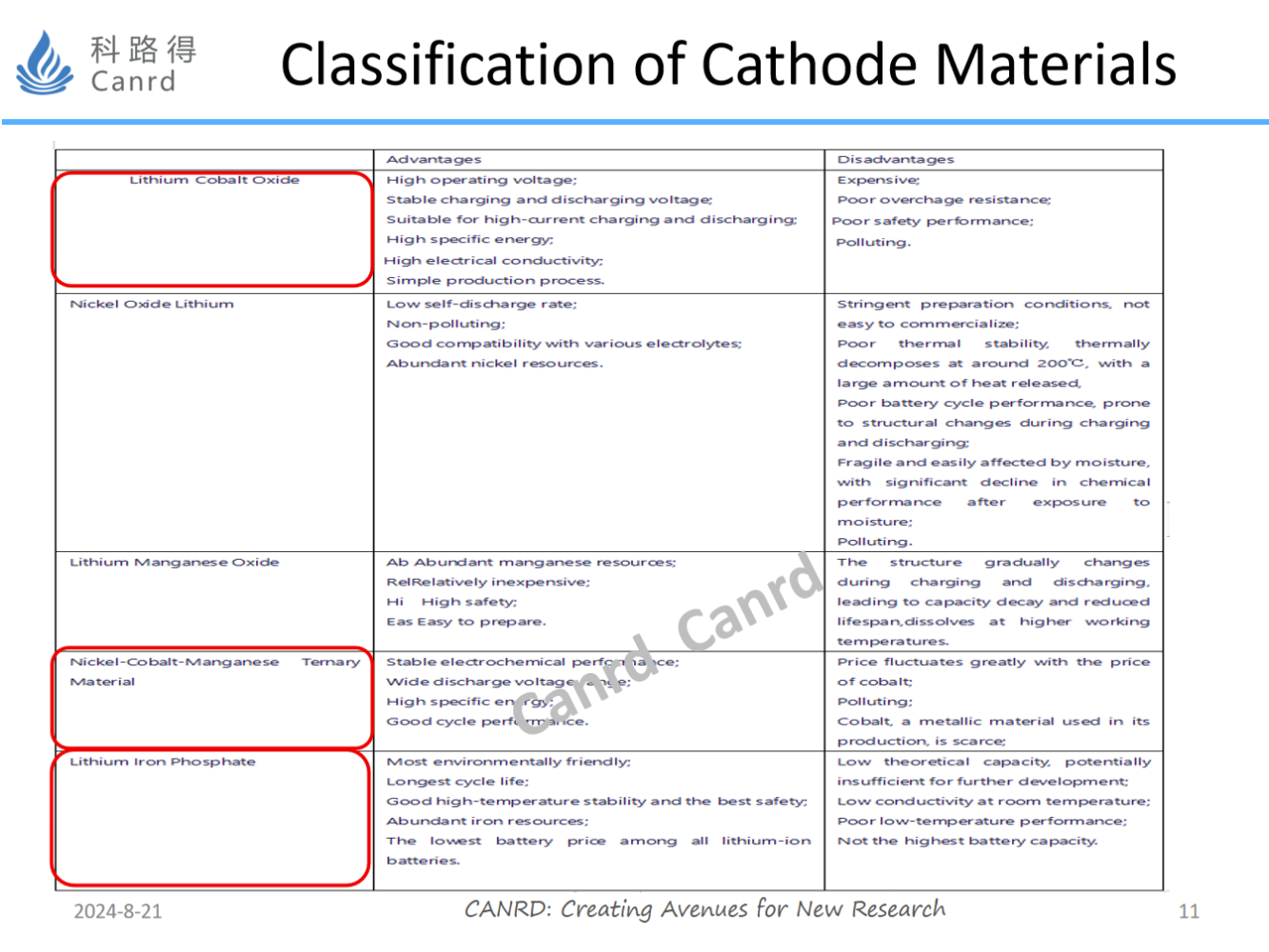
From this diagram, we can see the advantages and disadvantages of different materials, each material has its own characteristics, no one is good in all aspects, so it is necessary to choose different materials according to different application requirements. Lithium manganese oxide is used more in Japan and South Korea and is used in power batteries, but due to its low energy density, it is gradually replaced by NCM, or mixed.
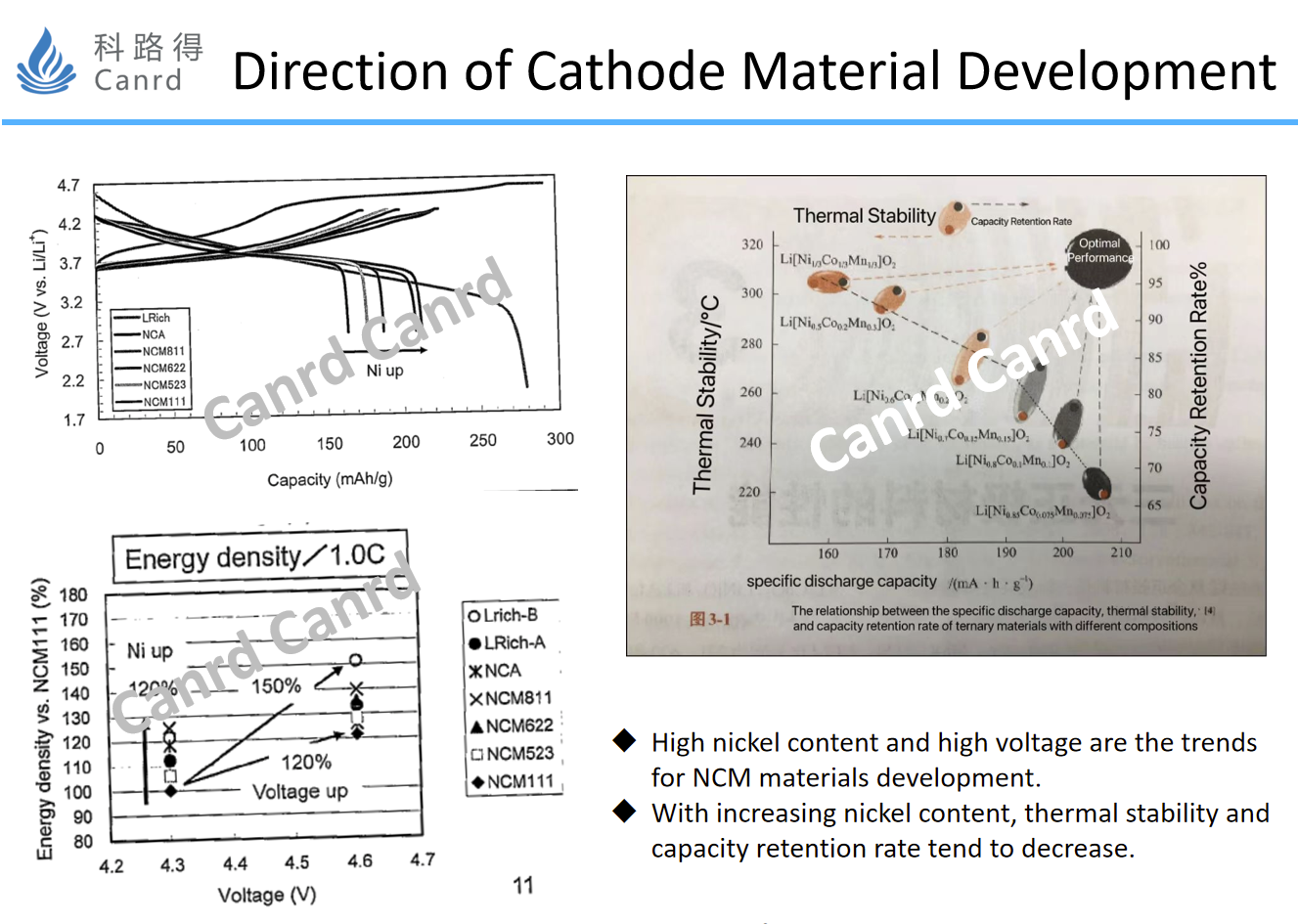
Here is the main introduction of NCM materials, currently two development directions, one is high voltage, the other is high nickel. As can be seen from the graph, the gram capacity increases as the nickel content increases, and as the voltage increases, both the gram capacity and the voltage platform increase. But as we can see from the graph on the right, an increase in nickel content leads to a decrease in thermal stability and capacity retention. Now that NIO has used the 811 battery, the energy density has been significantly improved, but the safety issues that come with it should also be paid enough attention. At present, the direction of lithium cobalt oxide is basically to increase the voltage, which has been increased from 4.2V to 4.5V, and the difficulty is getting bigger and bigger.
In addition to the conventional NCM materials, high-pressure nickel-manganese materials are also a hot spot in research. The nickel-manganese binary of 5V spinel has actually been studied for a long time, but because it requires high voltage to release more capacity, the performance of high voltage is not only the material, but also the electrolyte is also very important. Therefore, the development of electrolytes for such materials is also the key.
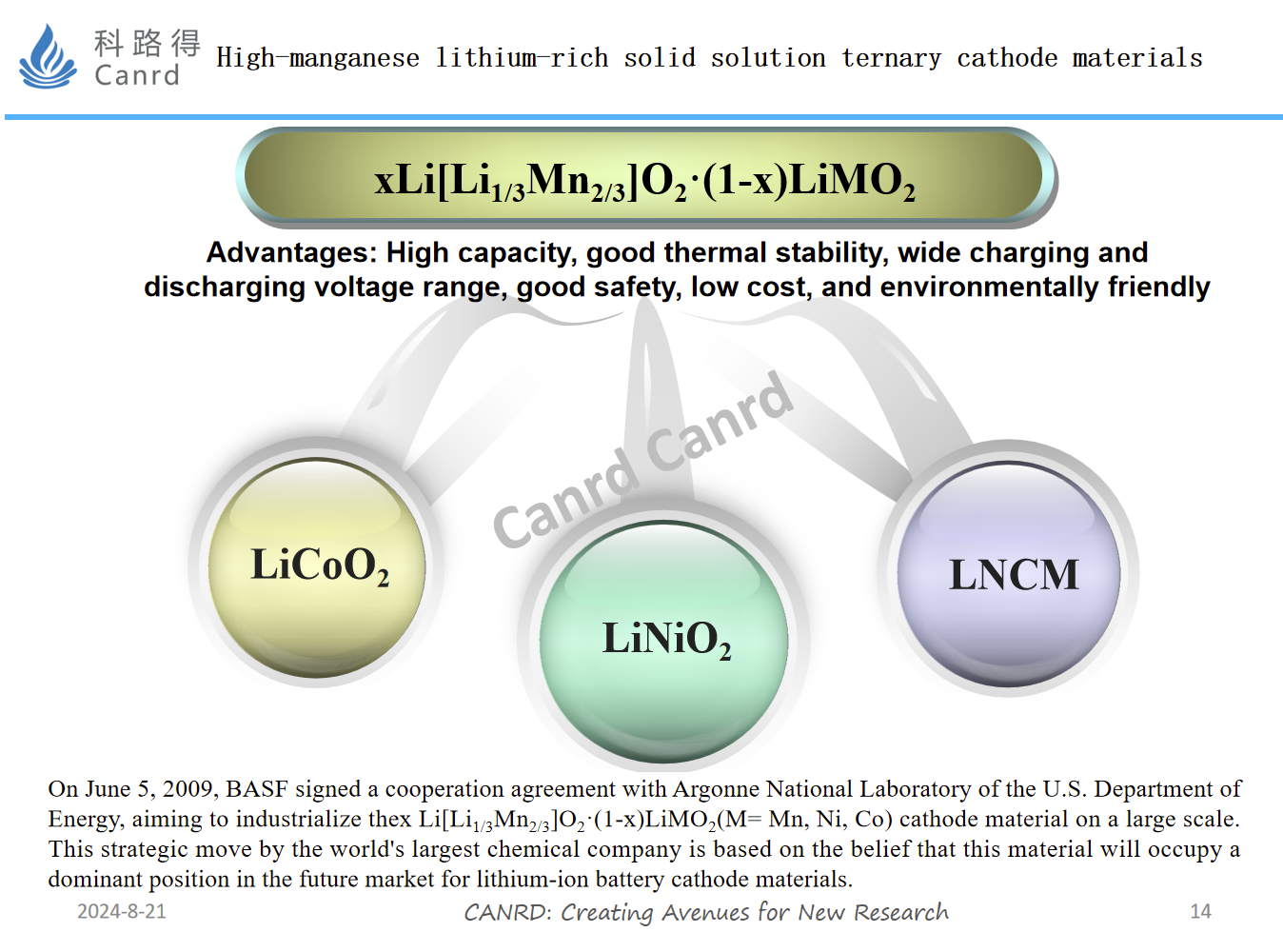
When lithium-rich manganese-based NCM materials were first developed, research was very hot, because the gram capacity was very attractive, reaching 250mAh/g. And Germany BASF and Japan Toda have carried out related industrialization work, but the result is industrialization. Nearly 10 years, this material has still not been commercialized. The biggest problem is the oxygen release and structural change during the first charge and discharge process, as well as the problem of continuous reduction of the voltage platform and poor cycle as the cycle progresses.
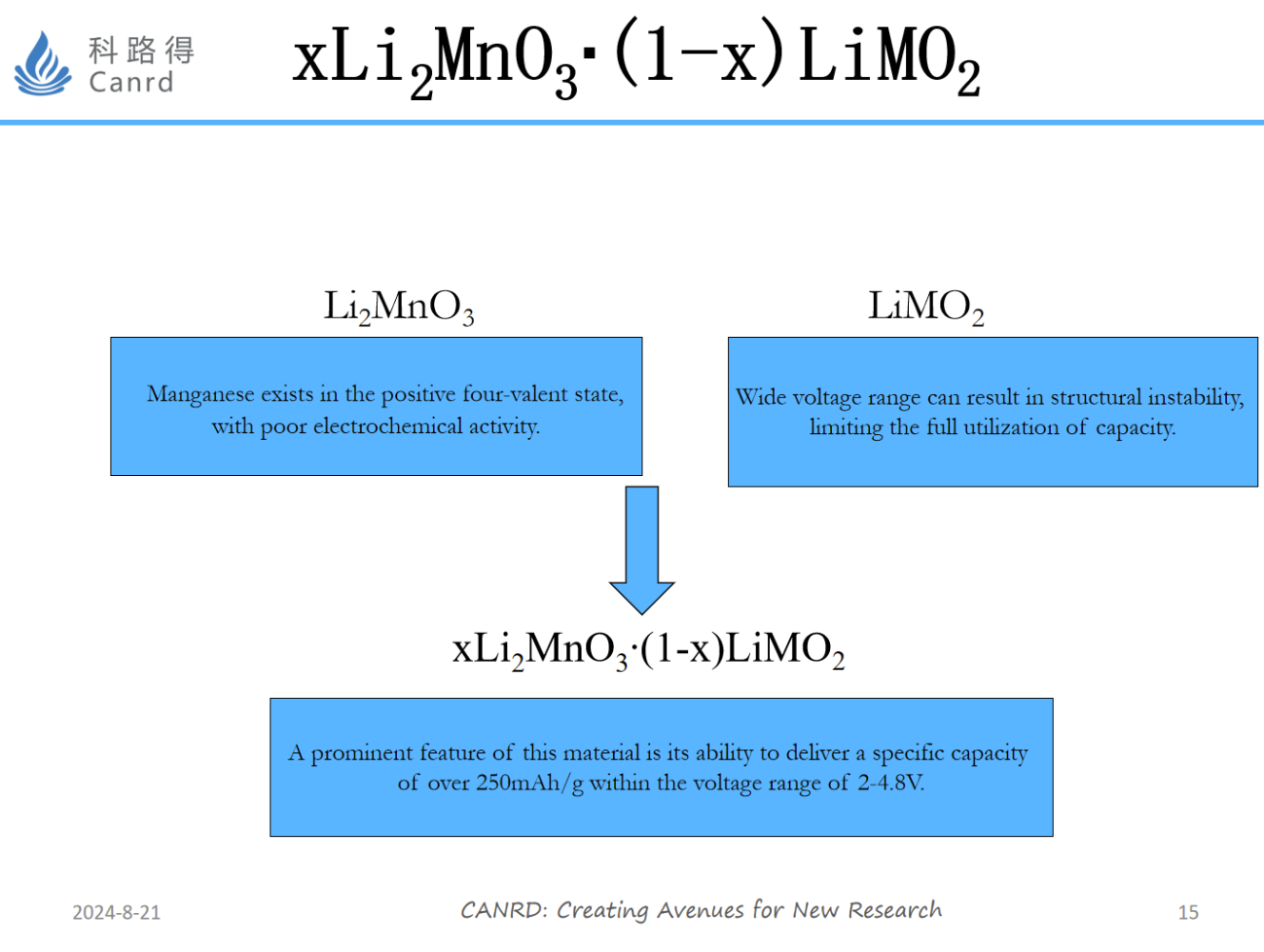
The material also needs to be at high voltage to give full play to the advantages of capacity, so the structural stability of the material and the matching of electrolytes are difficult to develop.
There are several attenuation mechanisms, and from the previous test results of pouch cells, it is indeed found that oxygen is released and the gas is finally formed.
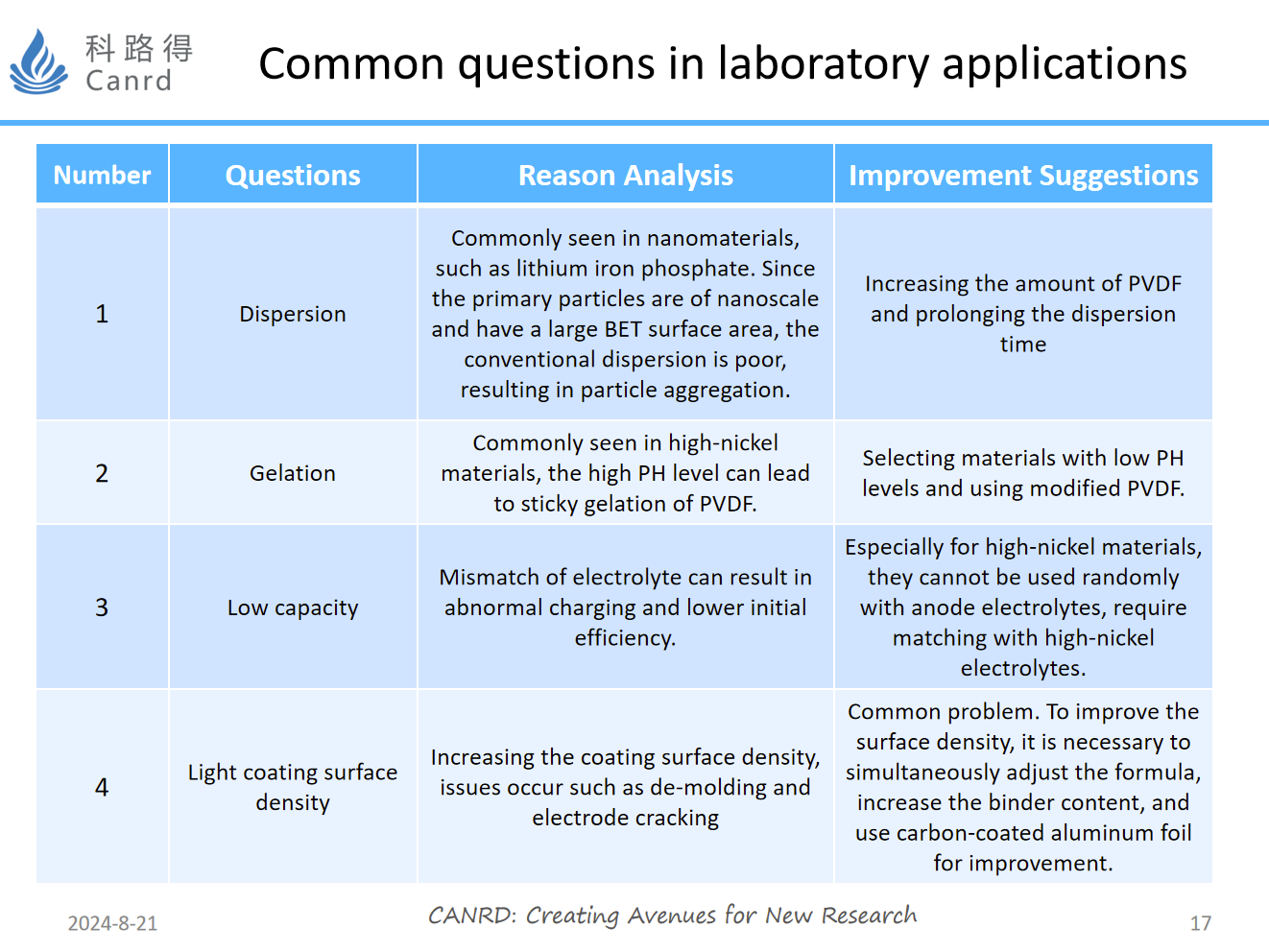
This is a collection of some of the most common problems, mainly dispersion, gel, low volume, and light areal density.
Due to the differences in the physical properties of different materials, it is often not possible to simply change the main material and the others remain unchanged when using materials. Nano-engineered materials require additional adhesives and longer dispersion times due to BET problems. Gels are generally prone to occur in high-nickel materials, which require targeted adjustment of the formulation and the use of modified PVDF. In addition, in terms of the use of electrolyte, the cathode and anode electrolytes cannot be mixed casually. In addition, we have also found that some cathode materials use the electrolyte of the anode electrode, which will continue during the constant voltage charging process, resulting in abnormal capacity.