
Here are all introductions to the anode material of lithium battery, which mainly stores lithium ions in the battery. In order to obtain a higher energy density, it is necessary to find an anode electrode with a low electrode potential on the one hand, and a higher capacity for storing more lithium ions, i.e. a low voltage platform and a high gram capacity. In order to ensure good cycling performance, the surface of the material needs to be stable, and the structure changes little during the charging and discharging process, so that the SEI film on the surface will be more stable.
Secondly, the material is required to have good electron and ionic conductivity properties, so that it has good rate characteristics. Finally, the material should be cheap, easy to make, in short, the price is low.
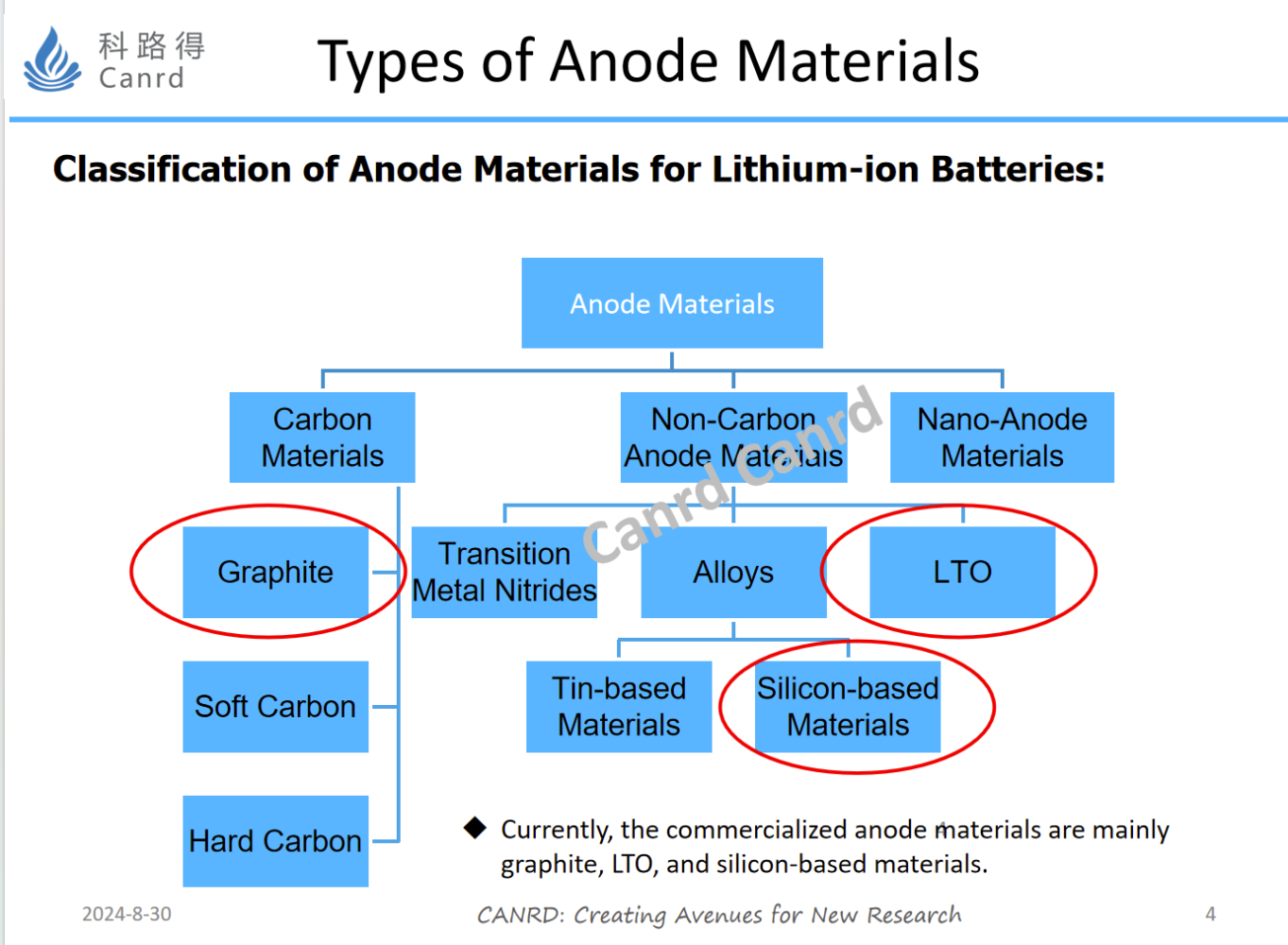
What are the categories of general anode materials? As you can see from the figure above, it is roughly divided into carbon materials, non-carbon materials, and nano-anode materials. Carbon materials are common graphite, hard carbon and soft carbon. At present, graphite is commercially used more, followed by LTO and silicon anode. Because of their high price and low energy density, hard carbon and soft carbon have only some applications in some power and fast charging products.
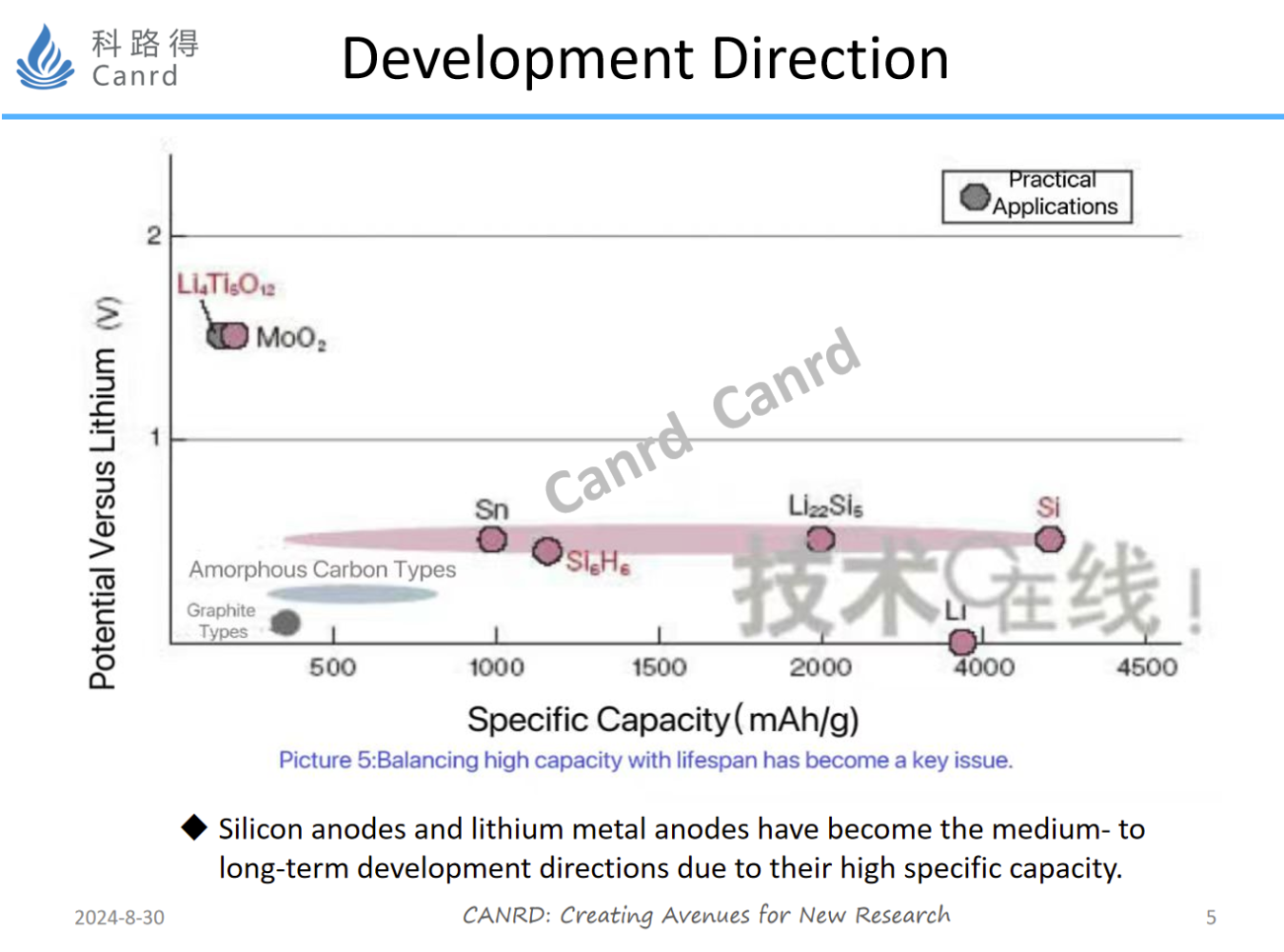
Borrowing a picture from the Internet here, we can see a development direction for commercial application of anodes. In order to further pursue higher energy density, it is necessary to increase the gram capacity of materials and develop them in the direction of Si, Sn, and lithium metal. At present, silicon anodes have been used in some applications, but they are still only low-capacity silicon-carbon materials, because the problems of expansion and circulation need to be further improved. In addition to high capacity, lithium metal anode also has the advantages of low voltage platform and light weight, but due to the problem of lithium dendrite, it needs to be improved with solid-state and semi-solid electrolytes.
Next, we will make some introductions to some mainstream anode materials.
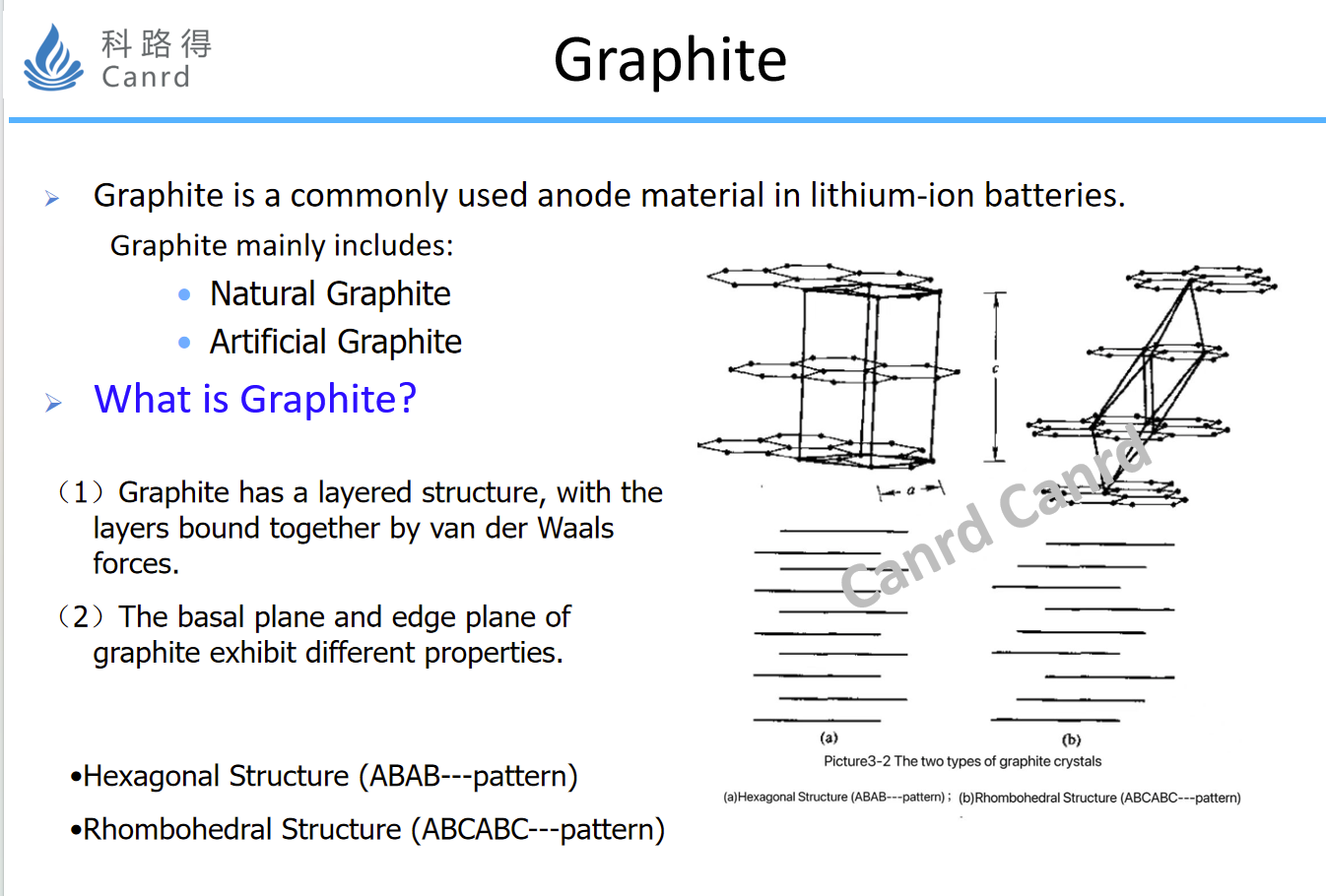
The first thing to talk about is graphite anode, which is the most widely used and longest-lasting anode material. It can be simply understood as the combination of many layers of graphene, combined with van der Waals forces, which is mainly divided into natural and artificial graphite. As the name suggests, natural is naturally formed and can be directly mined; Man-made is basically obtained by graphitization at 300 degrees. Graphite is divided into a base plane and an end face, the base surface mainly conducts electrons, and the reaction is carried out from the end plane with lithium intercalation.
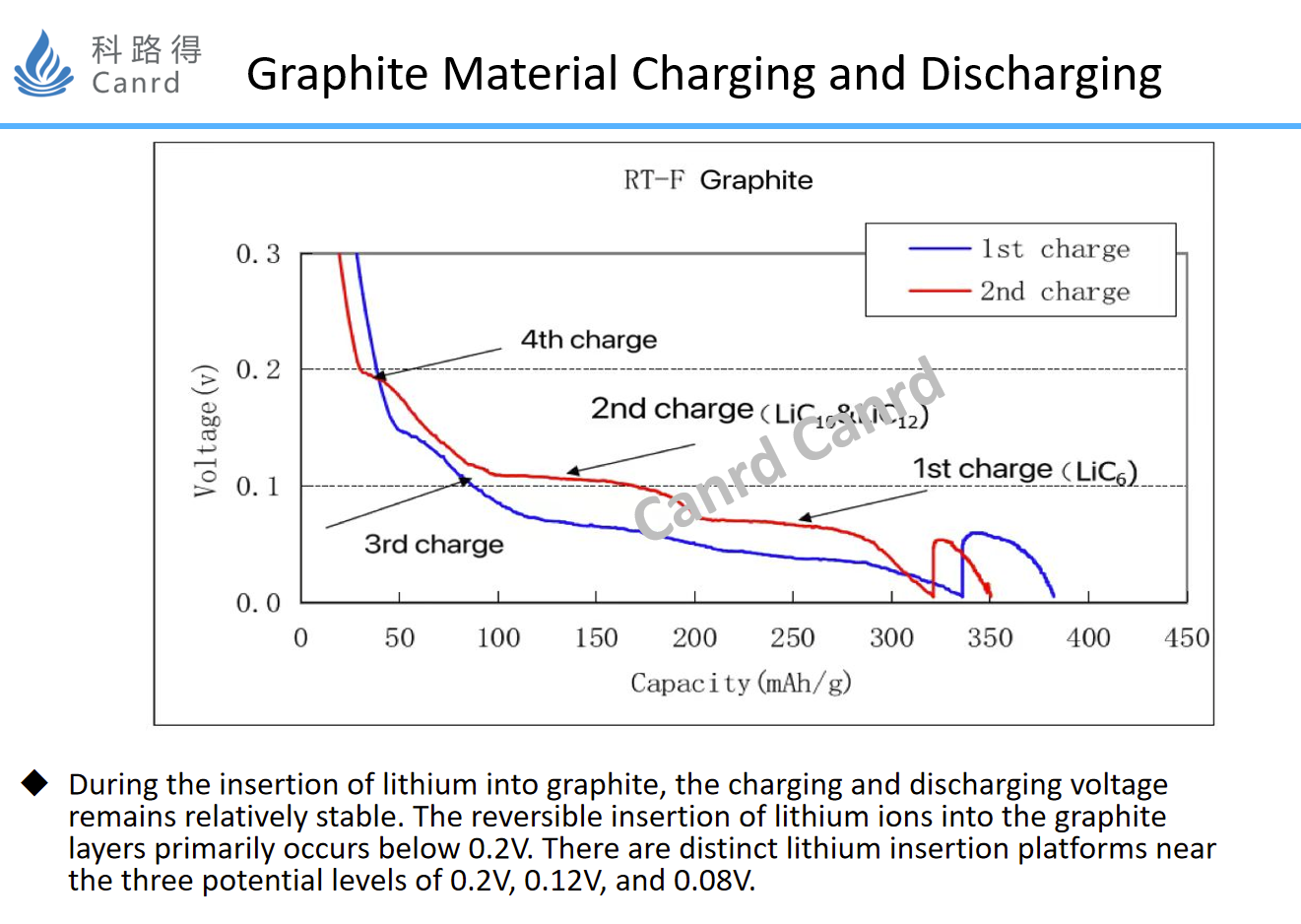
The discharge curve above is a standard graphite lithium intercalation curve, which is divided into four different lithium intercalation depths, and finally gets a full embedding of six-carbon lithium. This curve also shows that the graphite lithium intercalation process is carried out in an orderly manner.
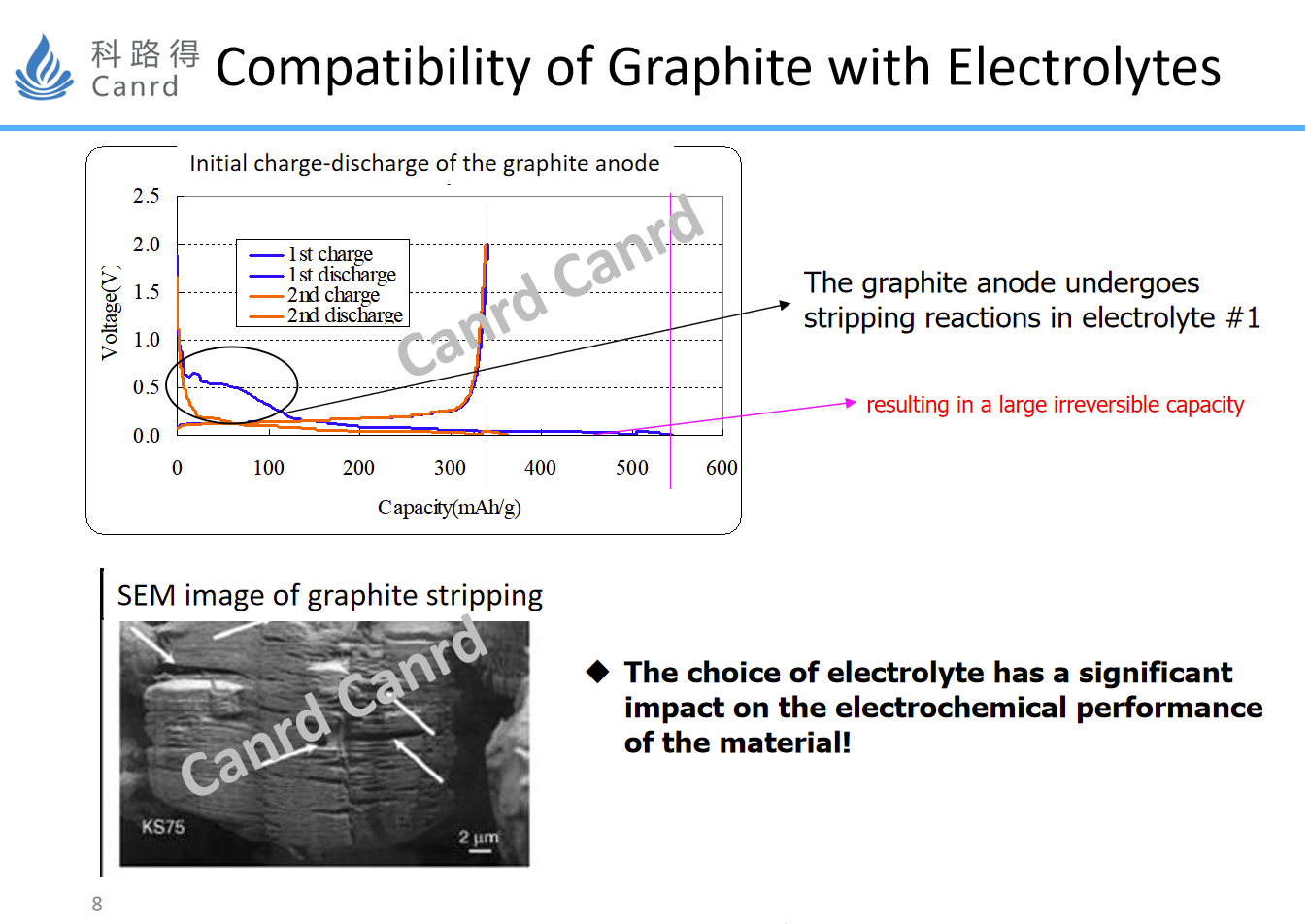
In the development of graphite anode, there were some technical difficulties in the early days. In particular, the electrolytes used in the early days contain PC solvent, which is very easy to destroy the lamellar structure of graphite in the early stage of SEI formation, resulting in the continuous side reaction of lithium ions, but they are not effectively embedded in the graphite layer. This is related to the structure of the PC and the resulting SEI film, which we will introduce in the subsequent electrolyte section. Therefore, the follow-up development of lithium battery is improved through two aspects: one is that the compatibility of artificial graphite to PC is better than that of natural graphite, and the second is that we have found that there are some additives that can be used to form a film-forming reaction and passivate graphite, so as to prevent the peeling reaction of PC, so that graphite materials can be used and developed significantly.
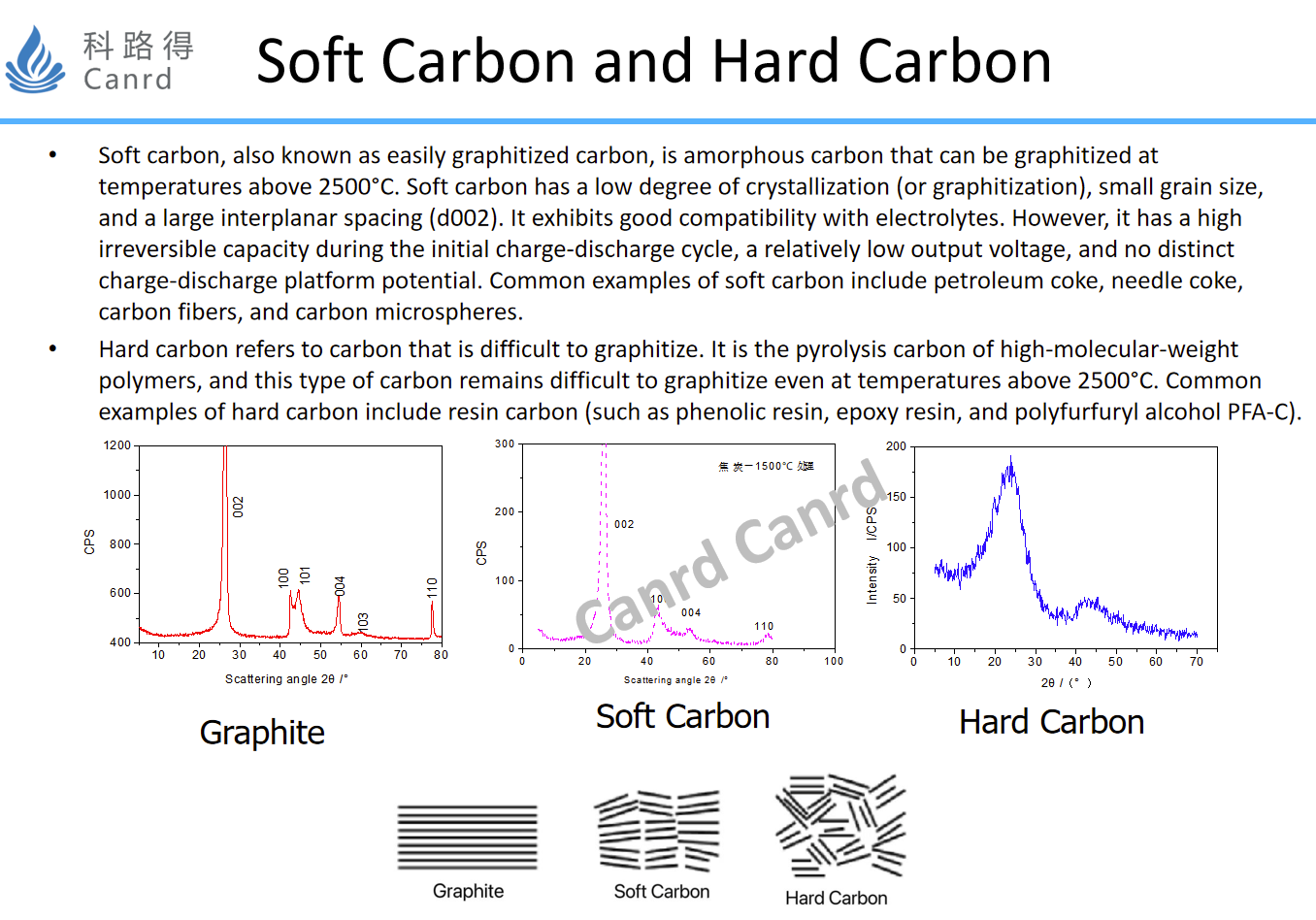
Both hard and soft carbon are carbon materials. Soft carbon is easy to graphitize, that is, when it reaches the graphitization temperature, it can be converted into graphite, while hard carbon is difficult to graphitize, that is, even if it is graphitization temperature, graphite cannot be formed because of structural problems. From the structural point of view, it is easier to understand its application, because the layer spacing between soft carbon and hard carbon is large, because the difficulty of lithium ion intercalation and detachment is small, and the rate performance is good, so fast charging and fast discharging have some advantages. In addition, the potential platform of these two materials is relatively high, so it is not easy to separate lithium compared to graphite.
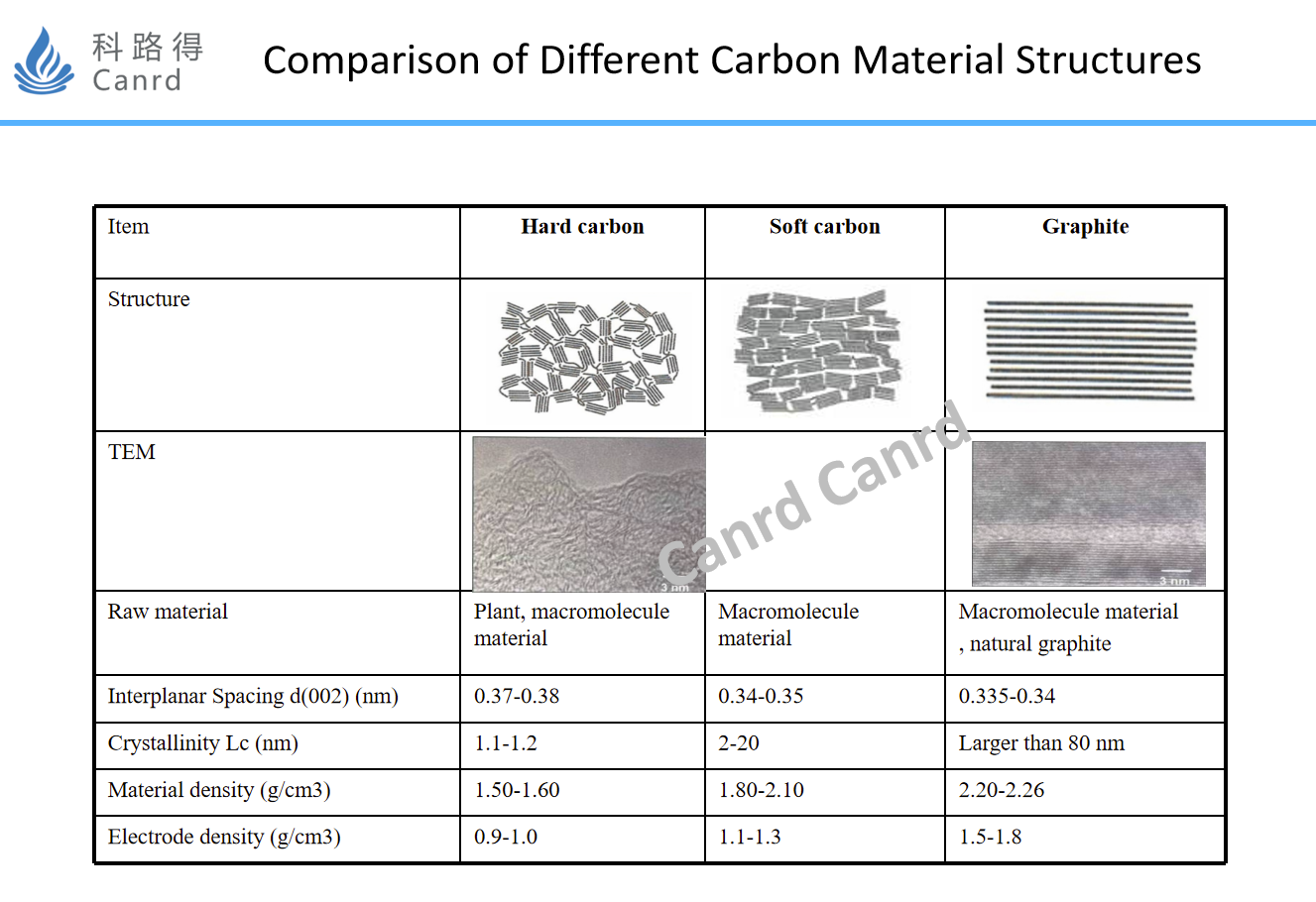
Here is a summary comparison of graphite and soft and hard carbon. The crystallinity can also be seen from the TEM, graphite is an ordered crystal structure, while soft and hard carbon is basically disordered. Soft carbon applications are very limited, and theoretically hard carbon should be best cycled with minimal structural changes.
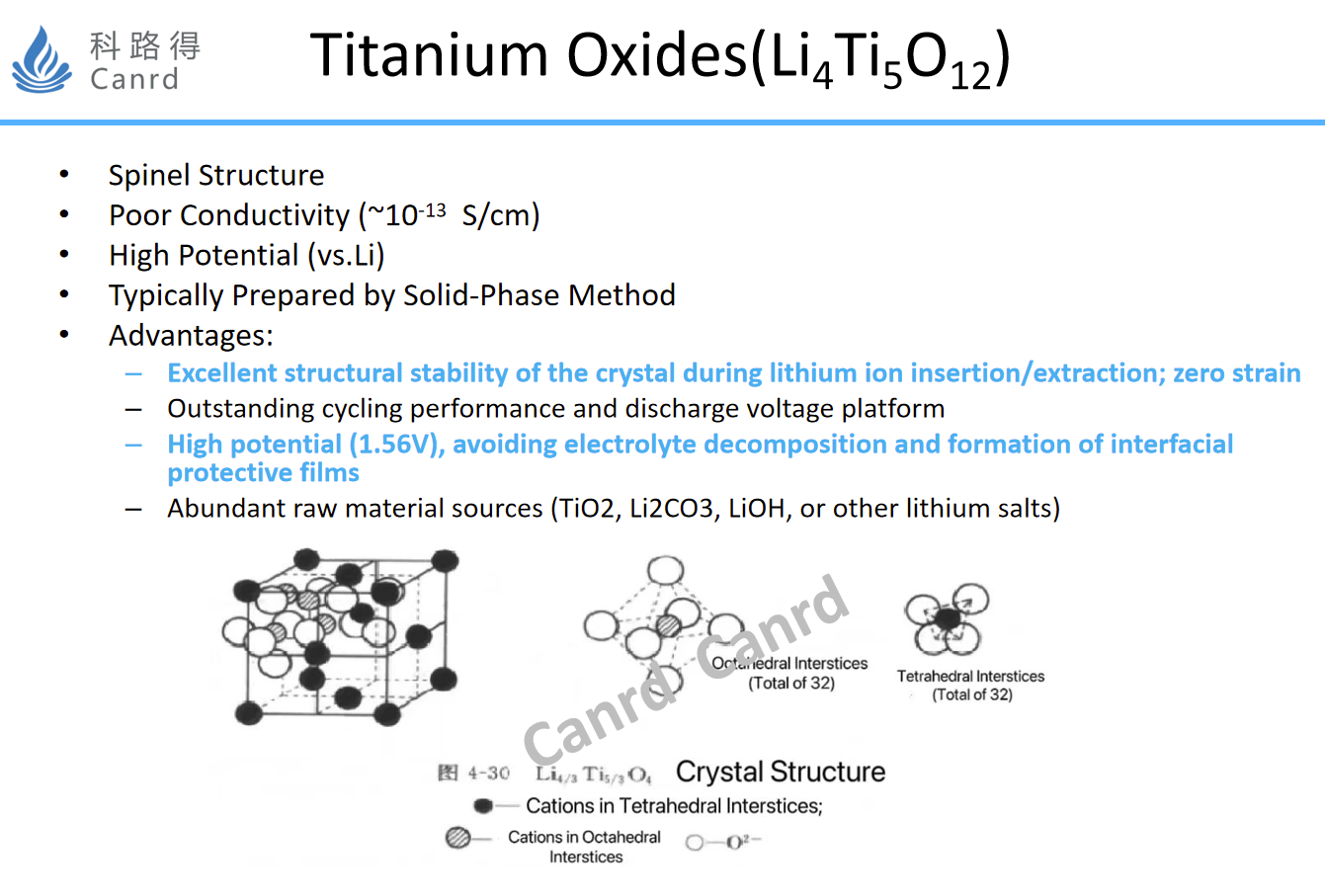
After talking about carbon materials, let's take a look at LTO, which is commonly known as lithium titanate. The advantages of this material are very obvious, O strain, high potential platform for charge and discharge, so the cycling performance is very good.
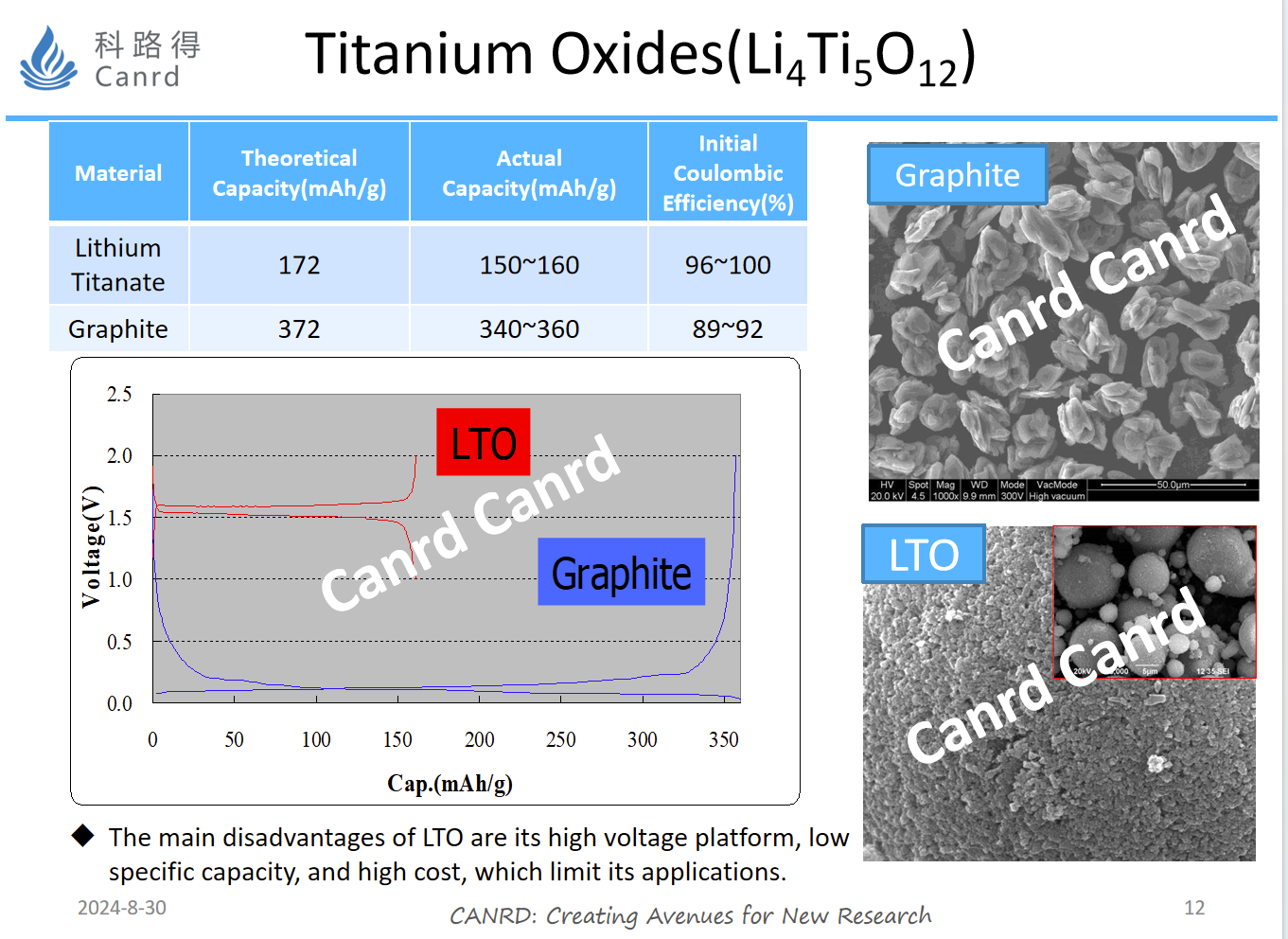
Dismantle under air humidity and will not catch fire. However, its disadvantages are also obvious, such as high potential and low gram capacity, resulting in low energy density, in addition, the cost of materials is relatively high. Therefore, it is mainly used in some electric buses, such as Yinlong's buses. In terms of energy storage, although the cycle and large rate performance are not bad, the price is relatively high, so it is difficult to apply.
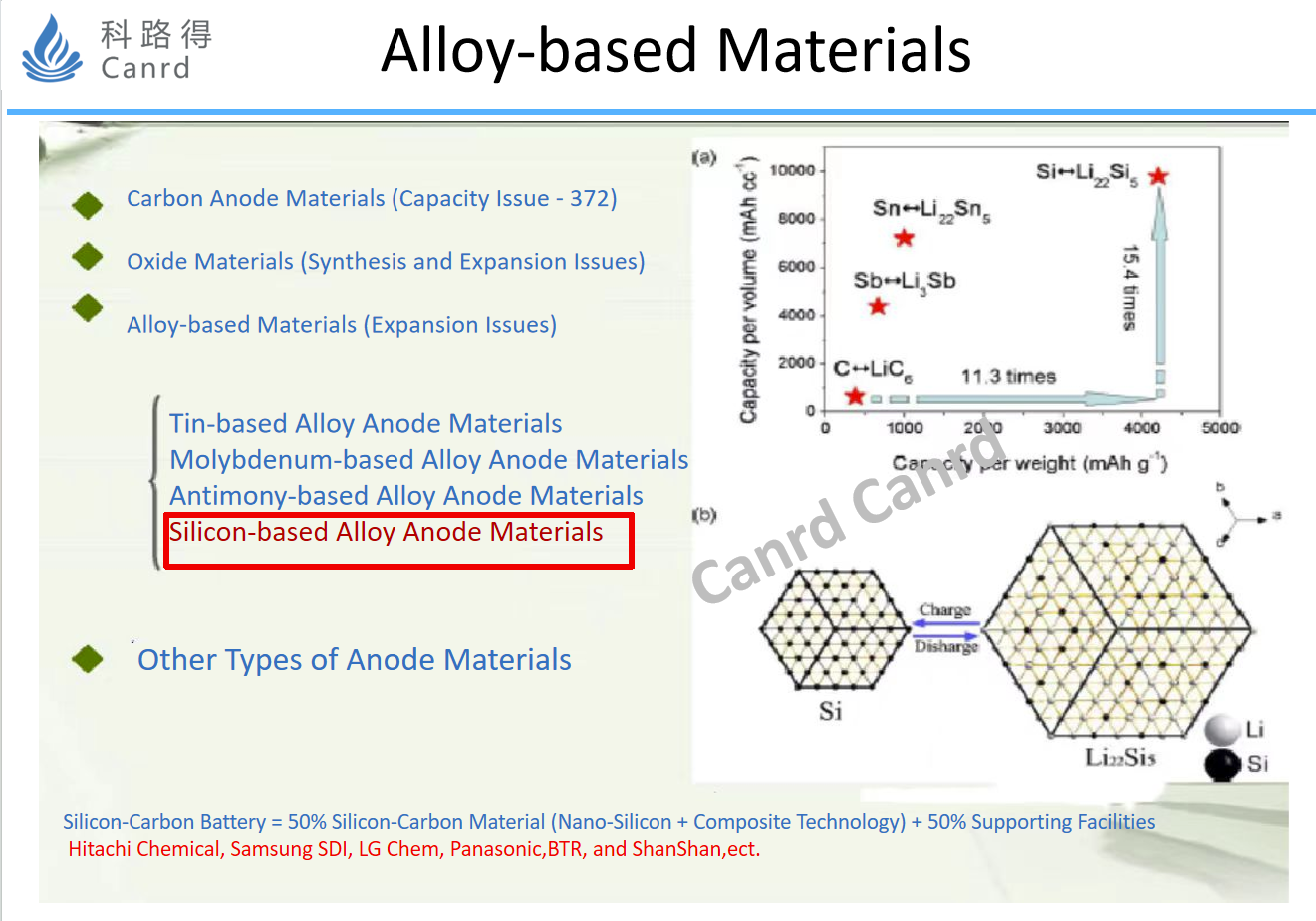
The next is alloy materials, although the research and application of silicon materials is very hot now, but in fact, sony has applied SnCoC alloy materials 10 years ago. But the problems of this alloy material are still common - large expansion, difficult to synthesize, and the capacity of Sn is much less than that of silicon.
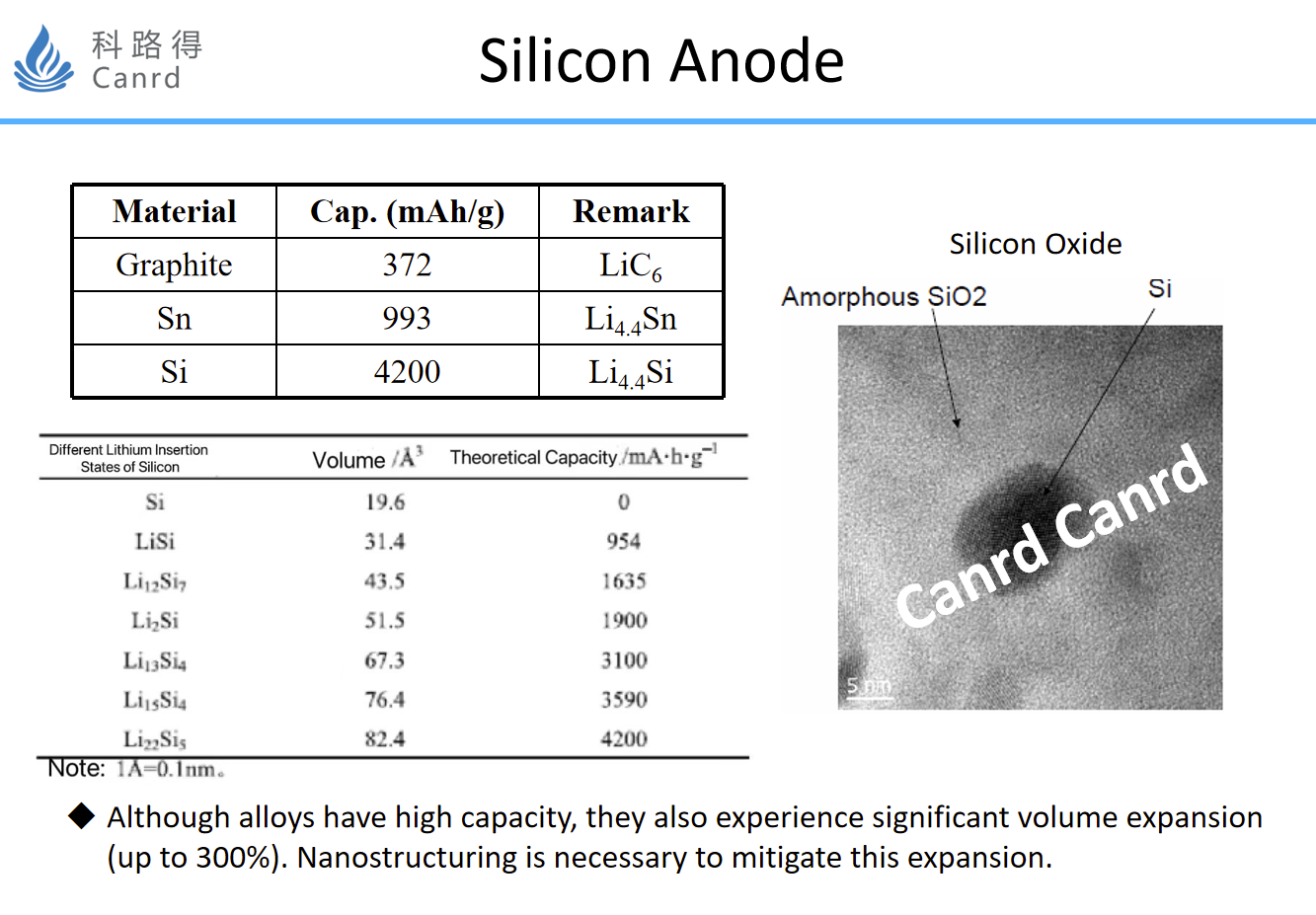
Let's take the silicon anode we are most familiar with, the capacity is theoretically 4200, which is much higher than Sn and graphite. Of course, because of thermodynamic reasons, in fact, the capacity measured by pure silicon is about 3500mAh/g, but this is already very high. In addition, we can look at the table in the middle of the figure above, where the volume of different lithium-silicon alloys varies from silicon to fully embedded Li4.4Si, and the volume expands by up to 300%. So, in the face of such a big expansion, the best option is nano. Some previous literature has reported that when the size of silicon particles < 150nm, there will be no chalking due to volume expansion during cycling. On the right is the TEM of the selected silicon-oxygen material, and the size of the silicon in it is within 10nm.
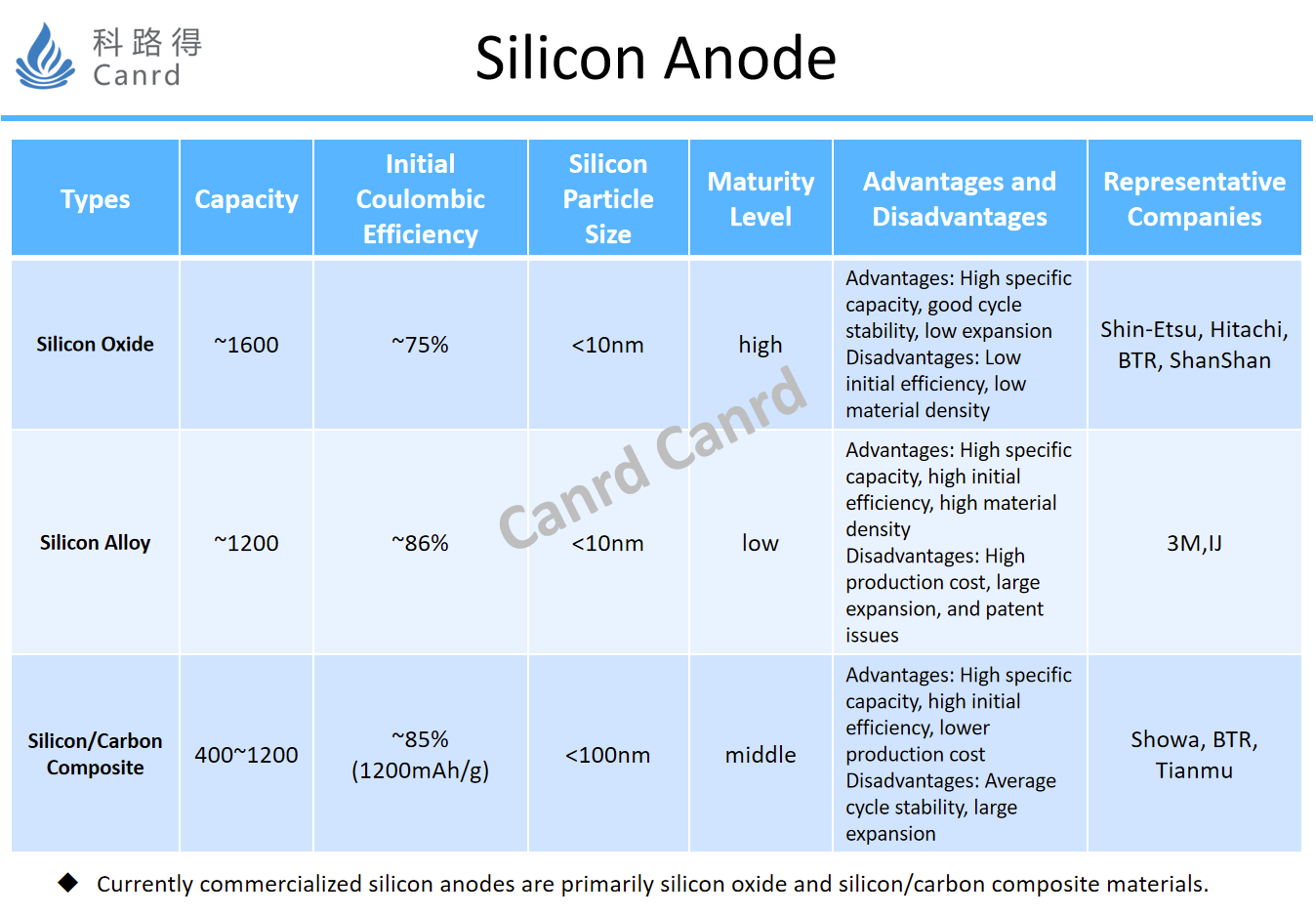
Here is a summary of silicon anode materials, and the characteristics of different materials are different. At present, due to the difficulty of synthesis and unstable structure of silicon alloy materials, 3M itself has given up industrialization. The other two materials, silicon-oxygen and silicon-carbon composites, are currently the mainstream. In terms of structural stability, silicon-oxygen is definitely the best, but its main problem is the low initial effect caused by the first lithium consumption, because the current addition amount is not much, and it is more suitable to match NCM materials with low initial efficiency. It is understood that the amount of Tesla added is about 10%. The advantages of silicon-carbon materials are that the capacity and first efficiency are high, and the generation process can be shared with graphite as much as possible, so the material cost will be lower, but the structural stability needs to be further improved. The first-class manufacturers of silicon anodes in China are mainly Beiteri, Shanshan, and Tianmu Pioneer, and the foreign ones are mainly Hitachi Chemical, Shin-Etsu Chemical, Showa and so on in Japan.