Today's course is mainly divided into three parts: analysis of process parameters affecting formation, introduction of process parameters of pouch cell formation, and introduction of process parameters of coin cel formation.
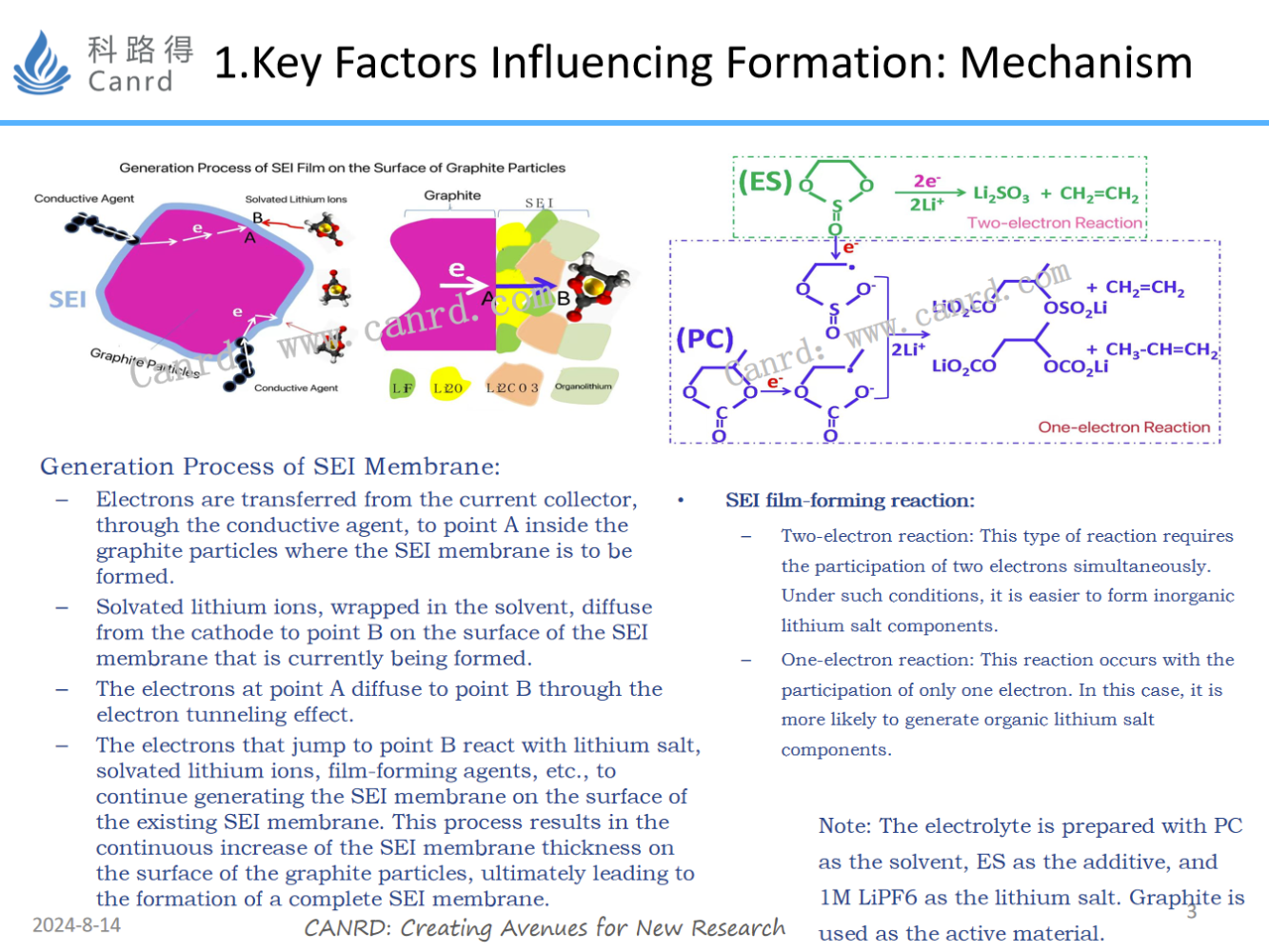
First, let's review the last lesson: the SEI generation process and the equation for the reaction. The SEI generation process is divided into single-electron reaction and two-electron reaction, and the SEI components generated by the two reactions are different, and the single-electron reaction mainly produces organic components; The two-electron reaction mainly produces inorganic components. The performance gap between different components of SEI is very large; Therefore, the SEI obtained by the single-electron reaction and the two-electron reaction is different.
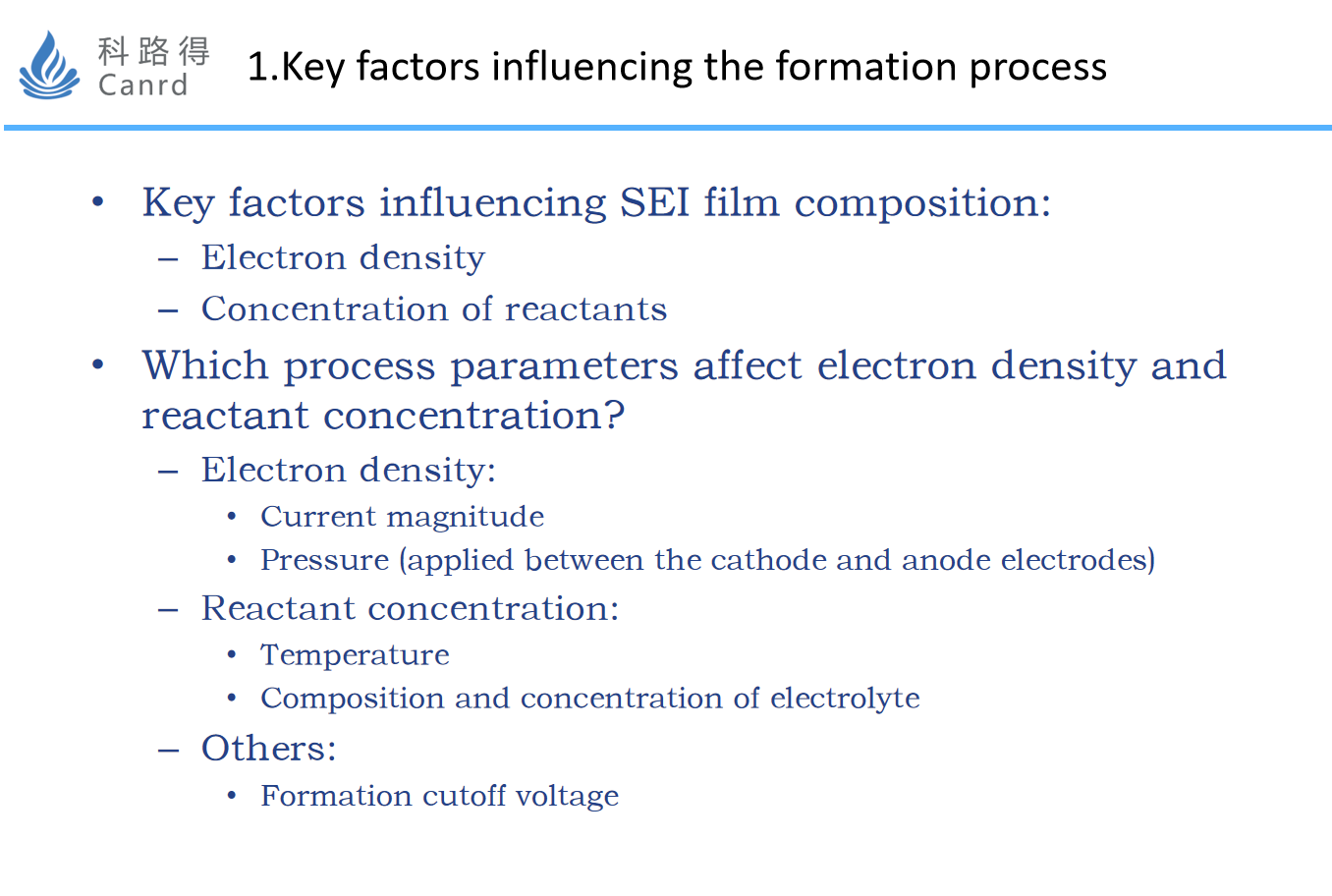
Summarizing the mechanism, it can be found that the key factors affecting SEI are: electron density and reactant concentration. It is then converted into process parameters, i.e., current density, reactant concentration, and others. The current density is mainly determined by the magnitude of the current and the uniformity of the current distribution. The reactant concentration is mainly determined by the electrolyte composition and temperature (polarization). Other factors include the formation cut-off voltage.
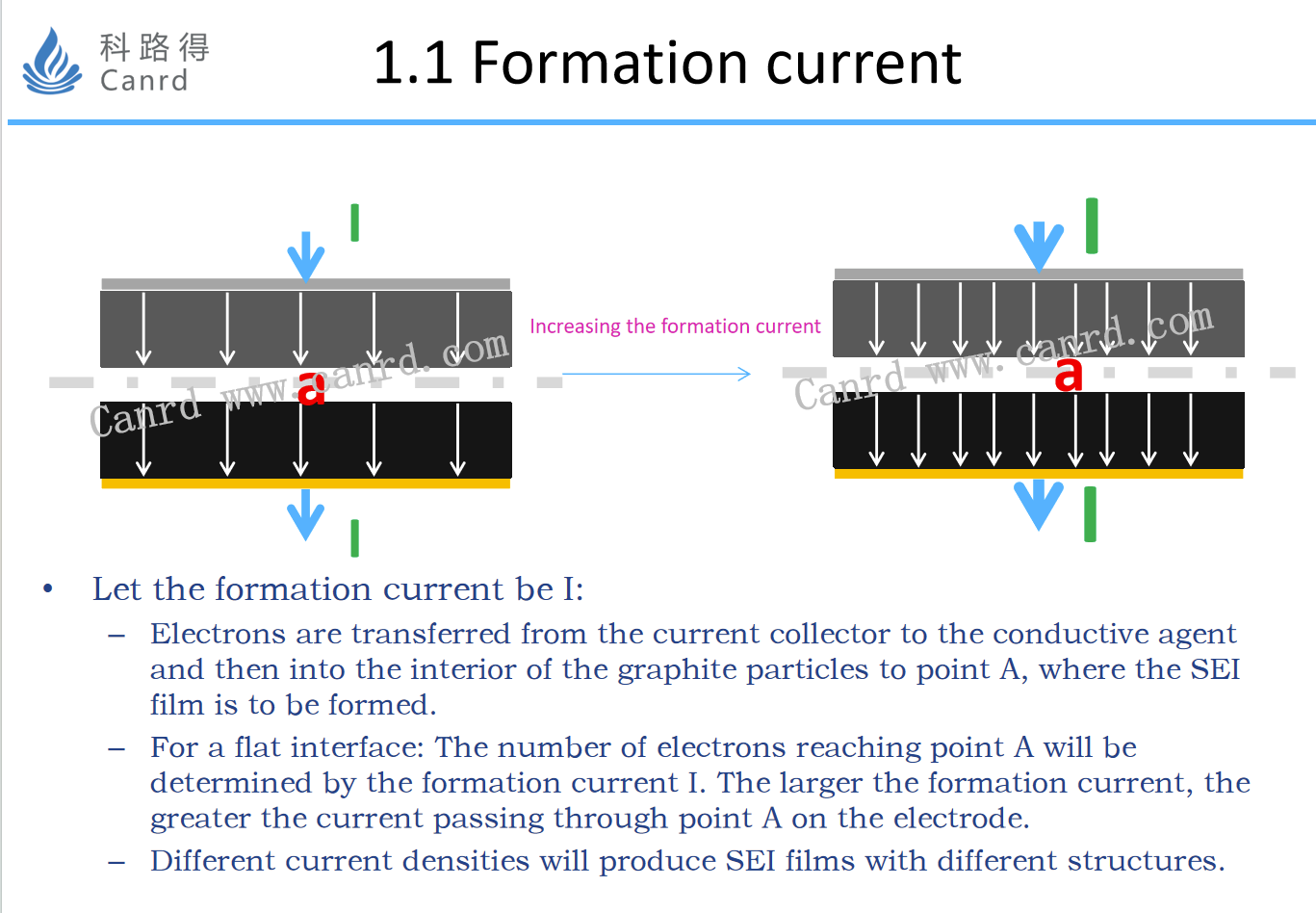
Formation current, the left and right sides are the schematic diagram of the formation current distribution of different current sizes. When the current density increases, the current reaching point a will increase, and the components of SEI production at point a will definitely change, which will have an impact on the performance of the battery.
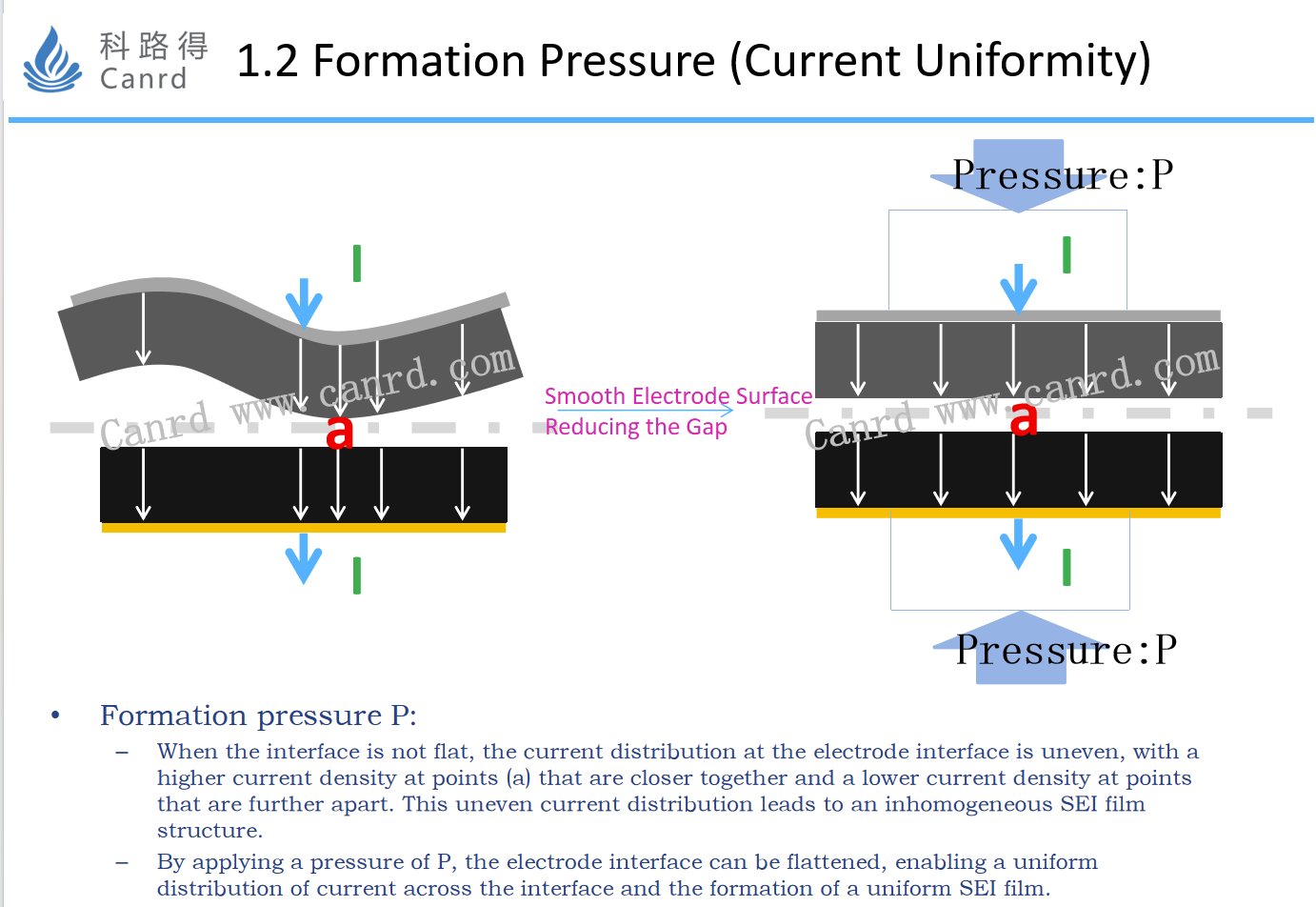
For the pressure of pouch battery formation, the most important thing is to improve the interface condition of the cathode and anode electrode.When the pressure P is applied, the interface will be flattened and the current will be evenly distributed between the two electrodes to form a uniform SEI film.
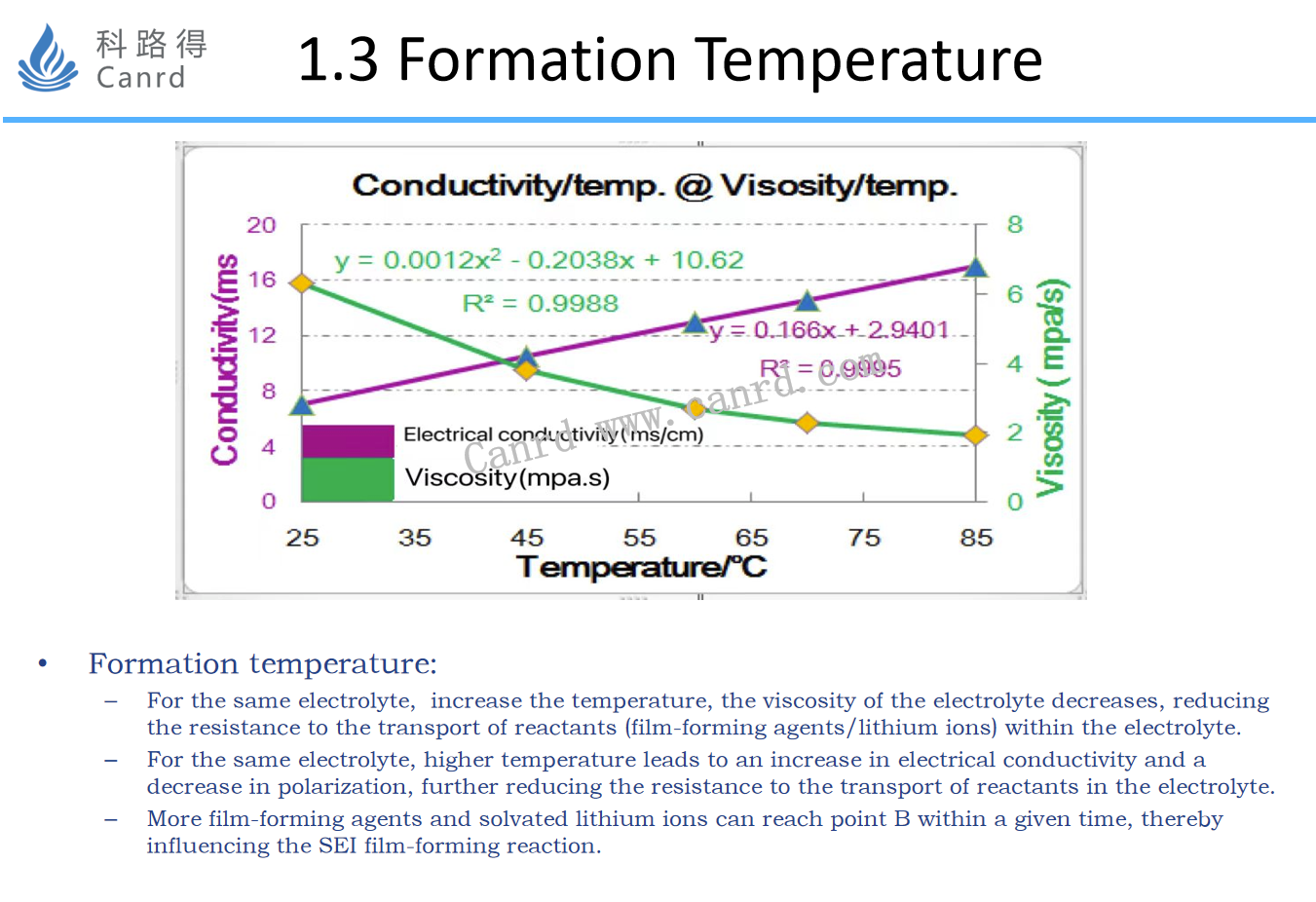
The formation temperature mainly affects the viscosity and conductivity of the electrolyte. As the temperature increases, the viscosity of the electrolyte decreases, and the conductivity increases, so increasing the formation temperature will reduce the polarization, which will change the concentration of reactants in the reaction to form the SEI film, thus affecting the production components of the SEI.
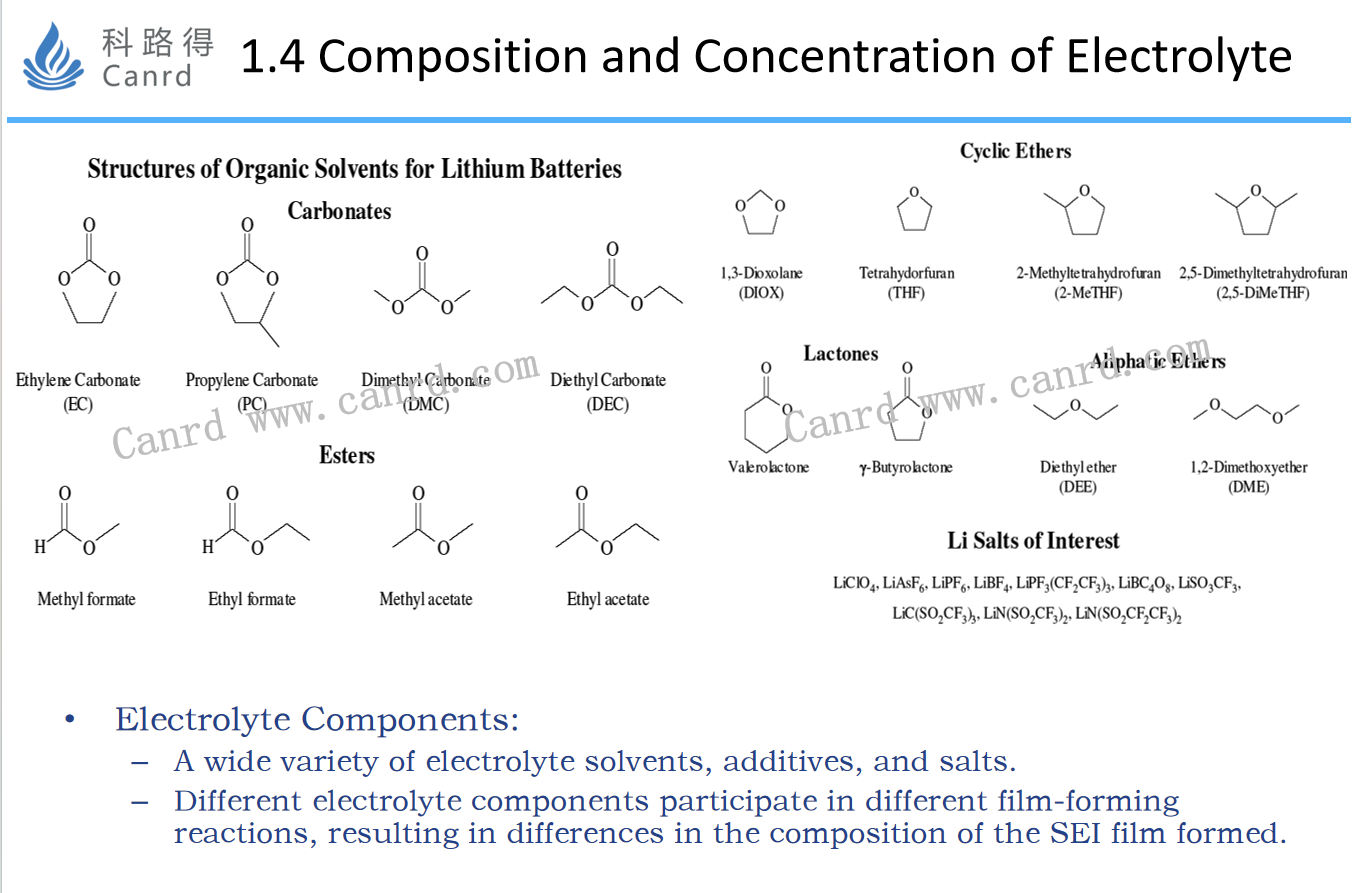
The composition and concentration of the electrolyte mainly change the type and concentration of reactants in the production reaction of the SEI film, thereby affecting the film-forming reaction of the SEI film, and ultimately affecting the formation effect.
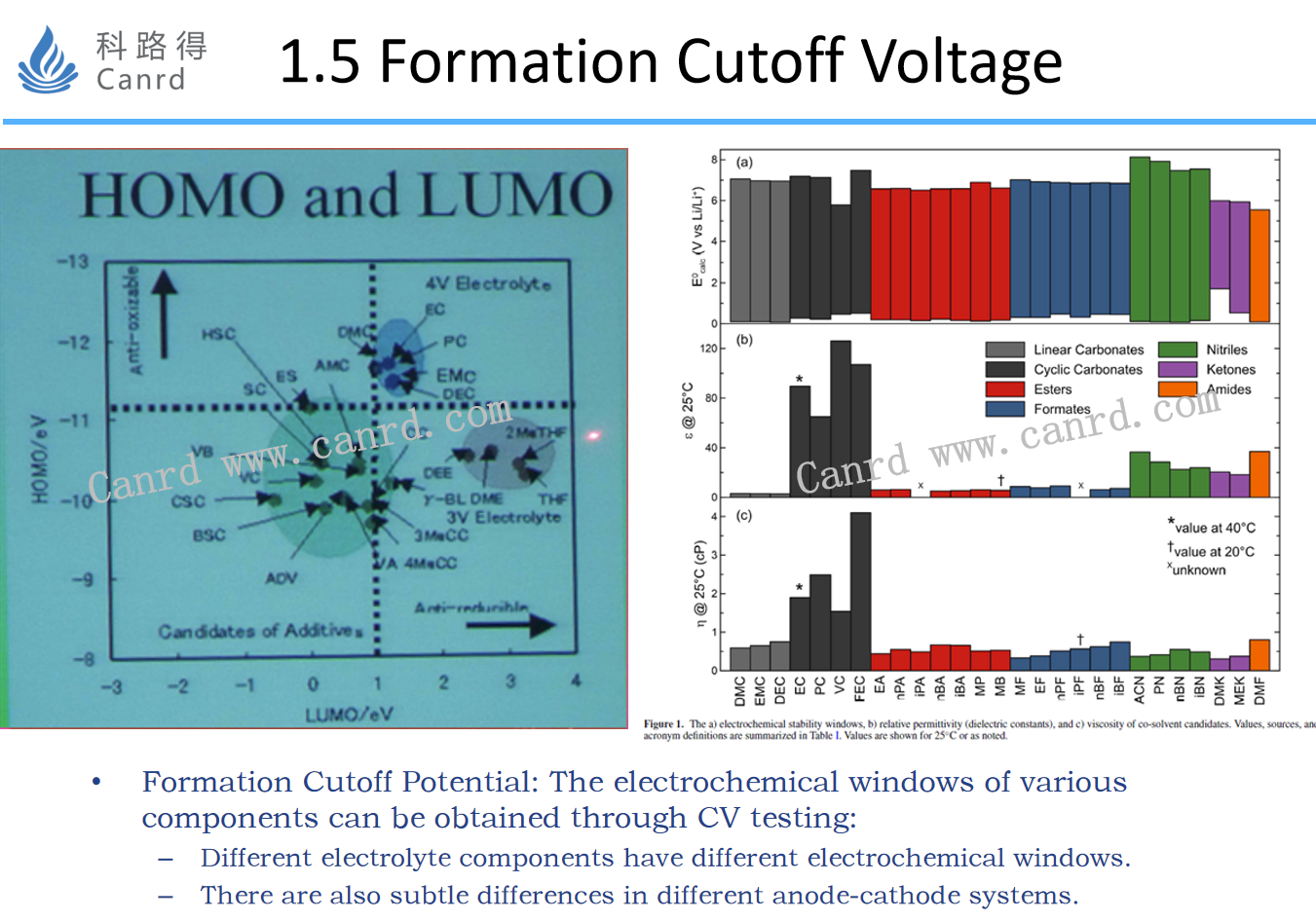
The formation cut-off voltage is mainly determined according to different electrolyte components, and the electrochemical windows of different electrolyte components are different, and the reaction potential is different, so the corresponding SEI film film-forming reaction potential.
The above five parts are the preliminary analysis of the influence of each factor on the formation effect. The following content is to give examples of the influence of these contents on specific process parameters in actual production. Let's continue to talk about the most used process in the industry for pouch battery formation: the clamp formation process.
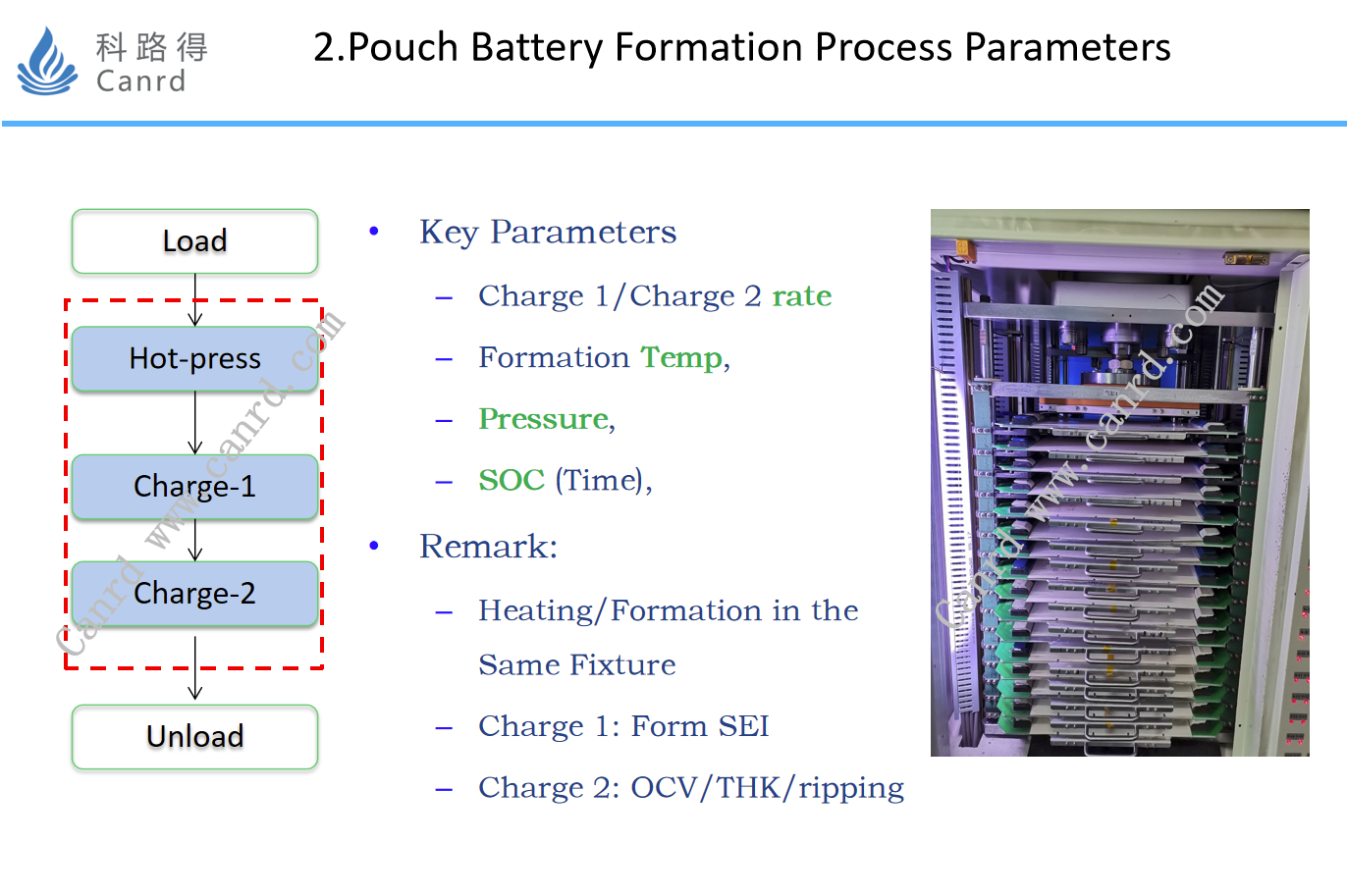
The figure on the right shows the more common vertical fixture forming machine: including heating, pressurization, and charging. The whole formation process, as shown in the leftmost flow diagram, is as follows: upper fixture, pressurization, hot pressing, charging 1, charging 2, and lower fixture. The key process parameters are: Charge 1, Charge 2, formation temperature, pressure, and SOC. Among them, charging 1 is to form SEI, and charging 2 is to charge the battery to a higher SOC, so as to control the open circuit voltage, thickness, deformation and other problems of the battery.
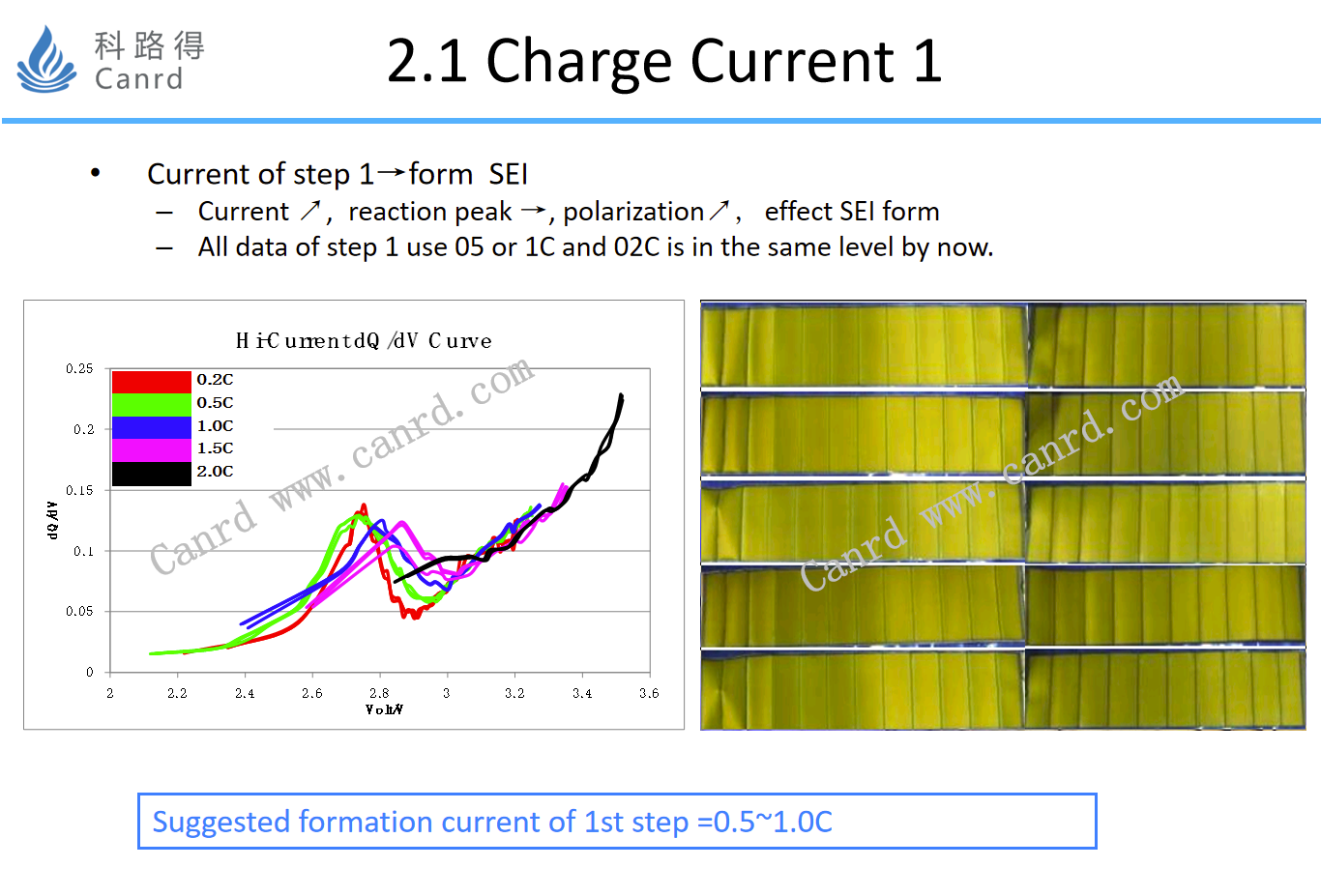
The charging current 1 is mainly to investigate whether the film-forming reaction peak is displaced; In this study, a dQ/dV-V curve was used, in which the position of the peak is the potential of the SEI film-forming reaction. Potential shifts, indicating significant polarization, will affect the performance of SEI.
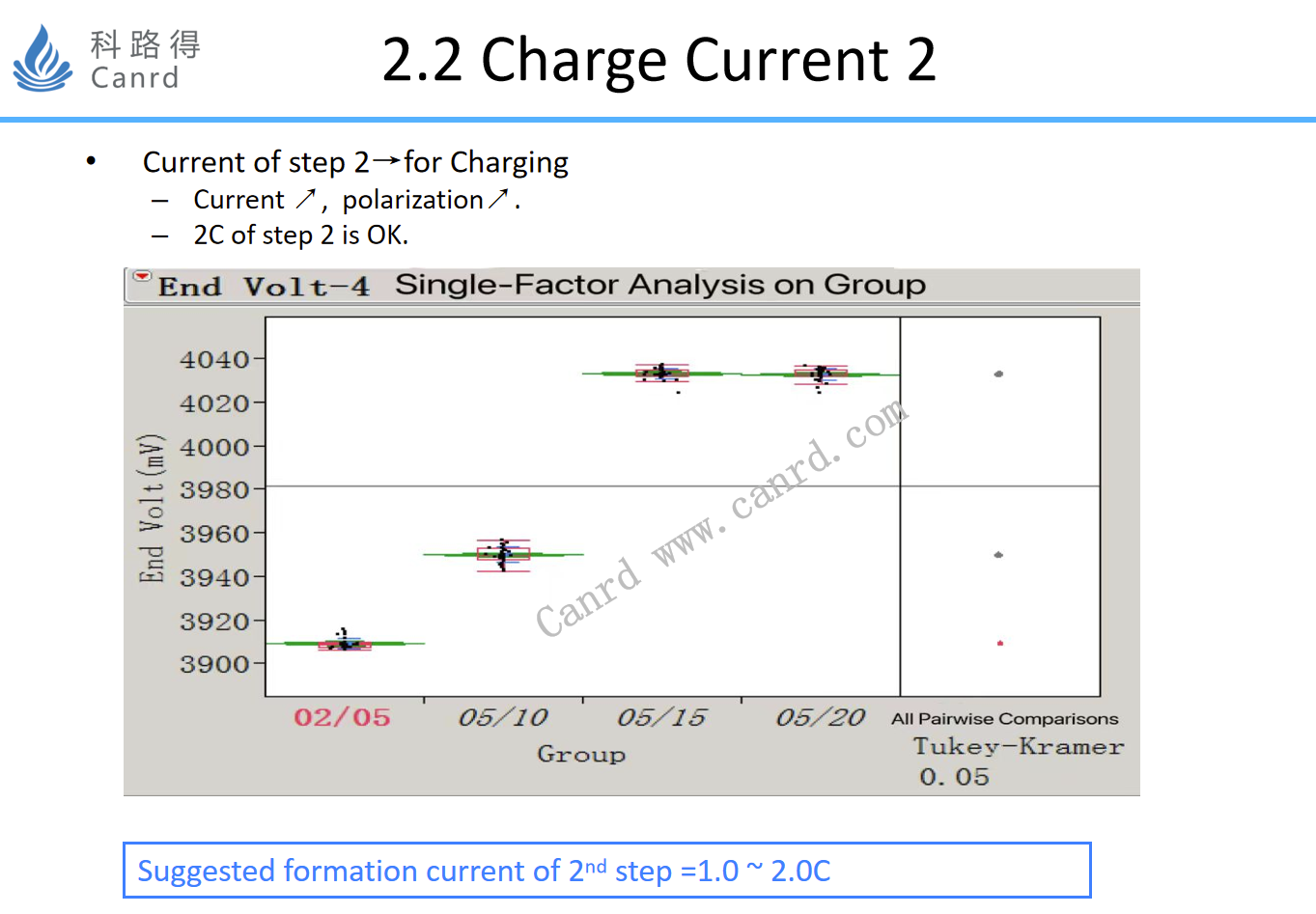
Current 2 mainly examines the polarization size, too large polarization will make the battery be protected, and the formation machine will stop automatically.
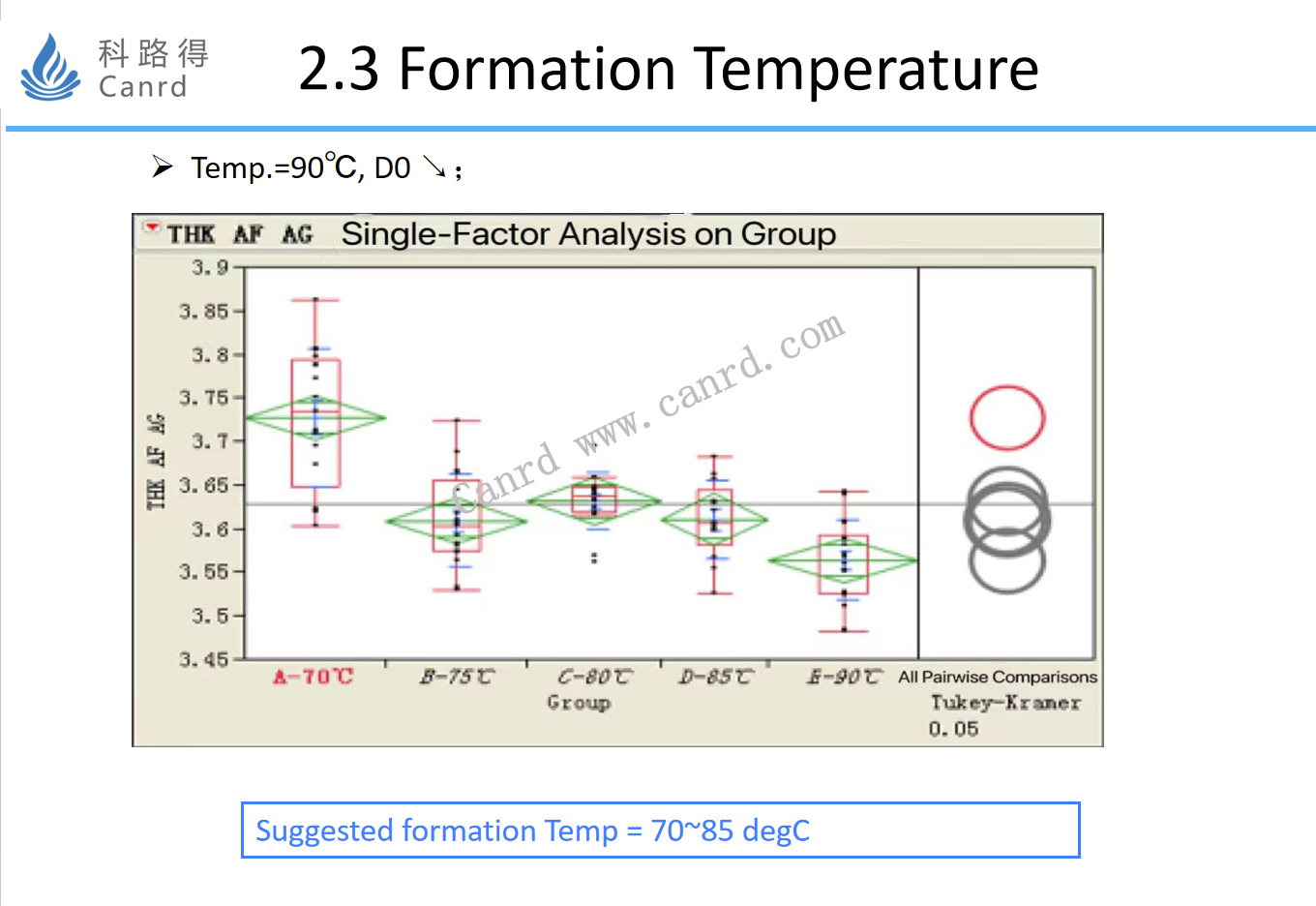
The formation temperature is mainly to investigate the effect on the capacity. Excessively high temperatures will reduce the capacity of the battery.
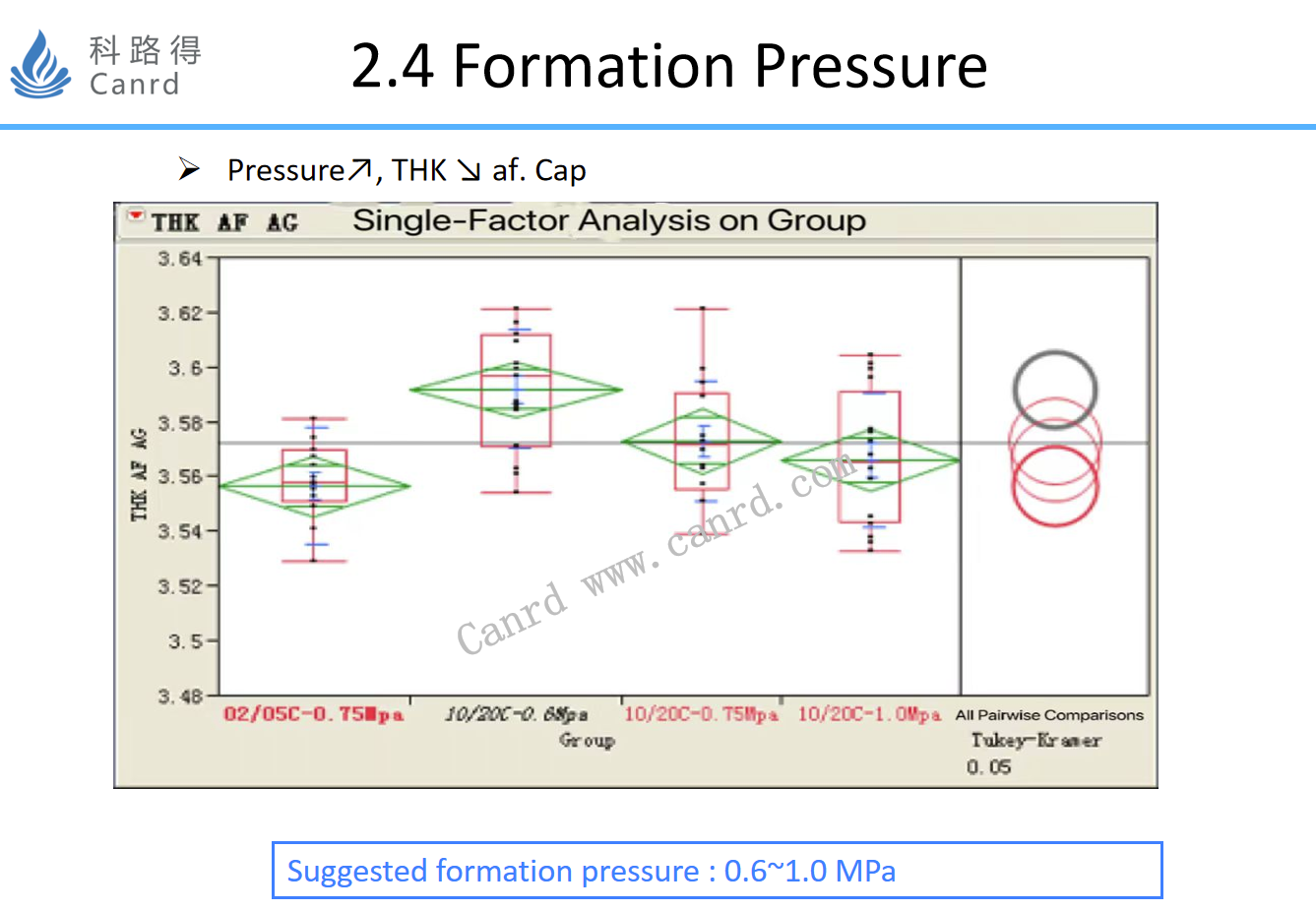
The effect of formation pressure is mainly to investigate the thickness of the cell after formation.
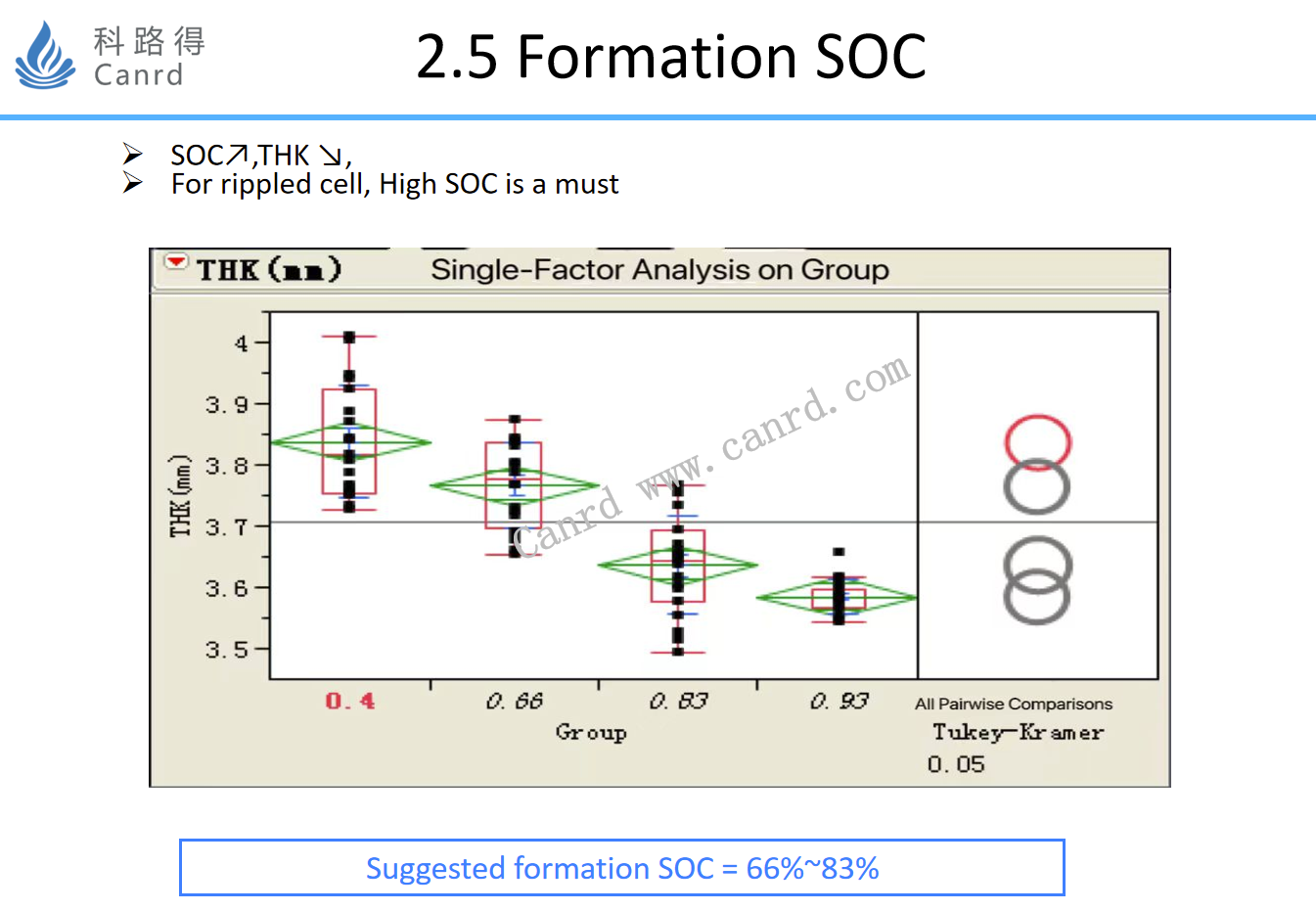
The formation of SCO is also to investigate the effect of formation on the final thickness of the battery. Of course, the adjustment of the above process parameters has other effects in addition to the above-mentioned effects; This needs to be systematically considered when it needs to be studied in detail.
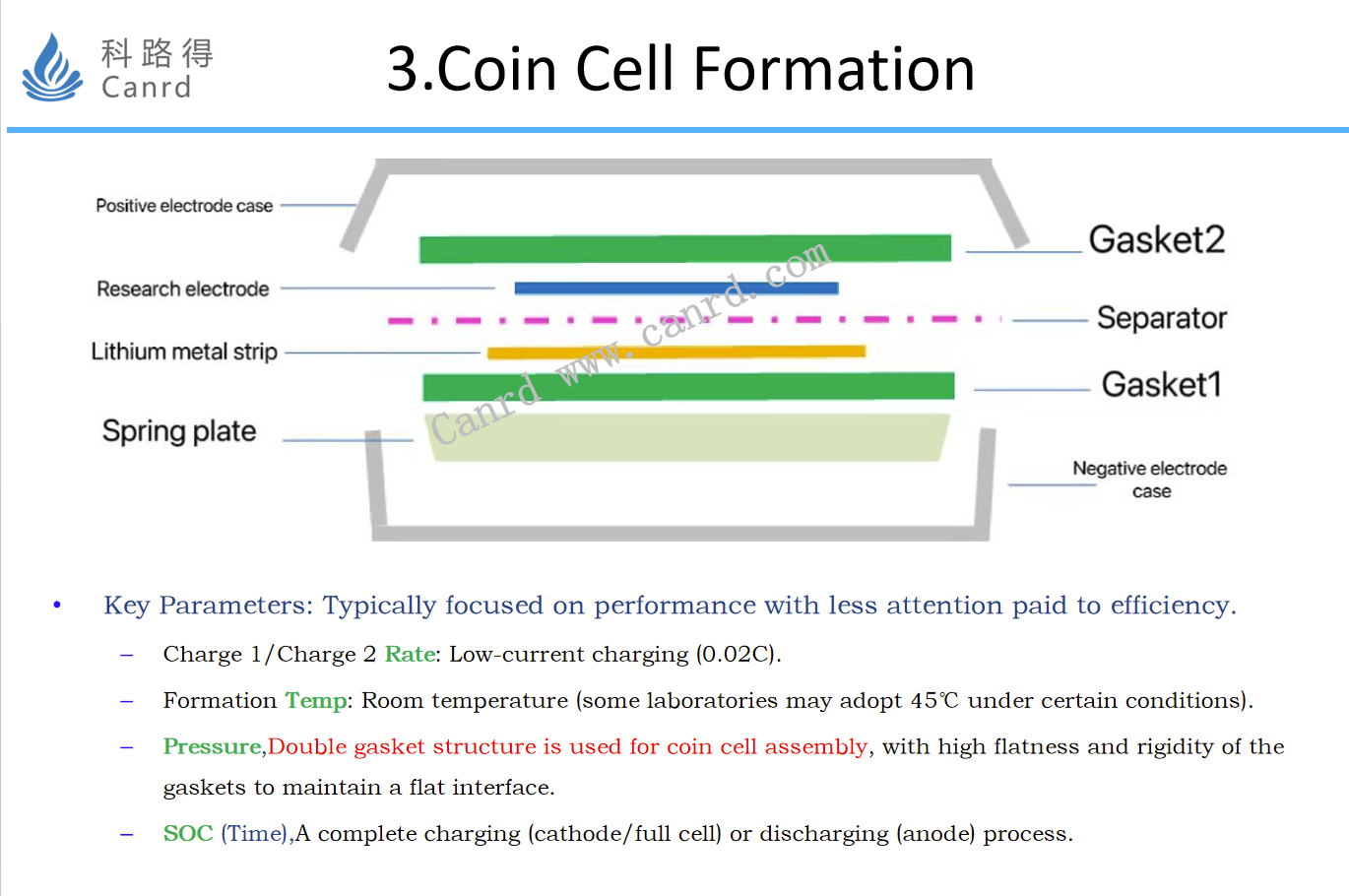
The formation of coin cell, first is coin cell assembly, and from the perspective of interface uniformity, we recommend the use of two gasket assembly structures, as shown in the figure. The main aim is to make the two electrodes absolutely flat. The biggest problem with this structure is that there is an extra gasket. For the formation current, 0.02C is recommended, because the formation of coin cell is basically done at room temperature. If there is a formation at high temperature, the formation current can be increased.
For the formation SOC, because the laboratory does battery research, the efficiency issue is basically not considered. Therefore, a complete charging or discharging process can be done.
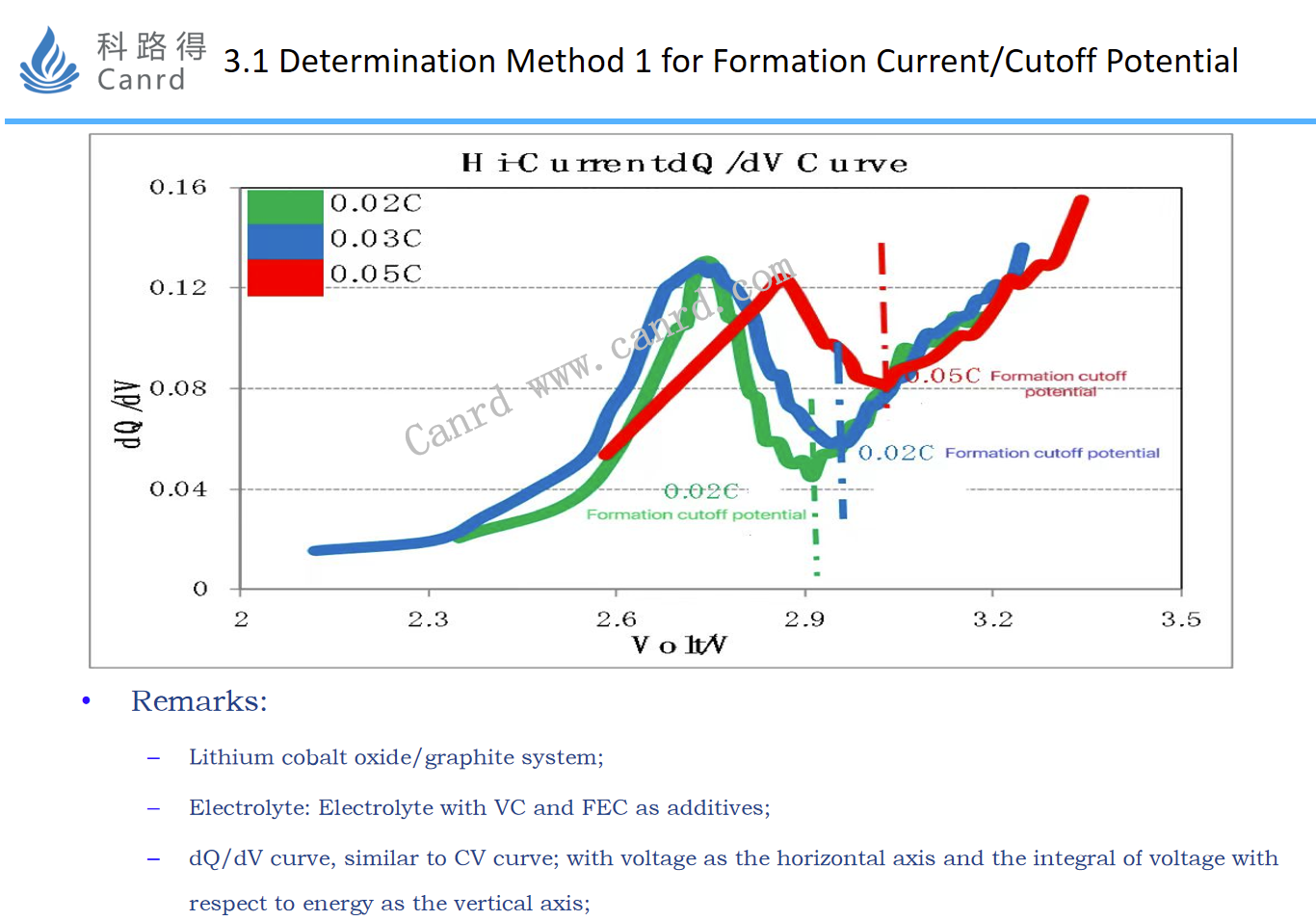
Similarly, the main method for studying the magnitude of the formation current is also through the dQ/dV-V curve.
Three formation currents of different sizes were made to investigate the displacement of the peak of the formation SEI film-forming reaction: 0.02C and 0.03C basically overlapped, and the film-forming reaction was basically the same, and the right shift at 0.05V indicated that the polarization increased.
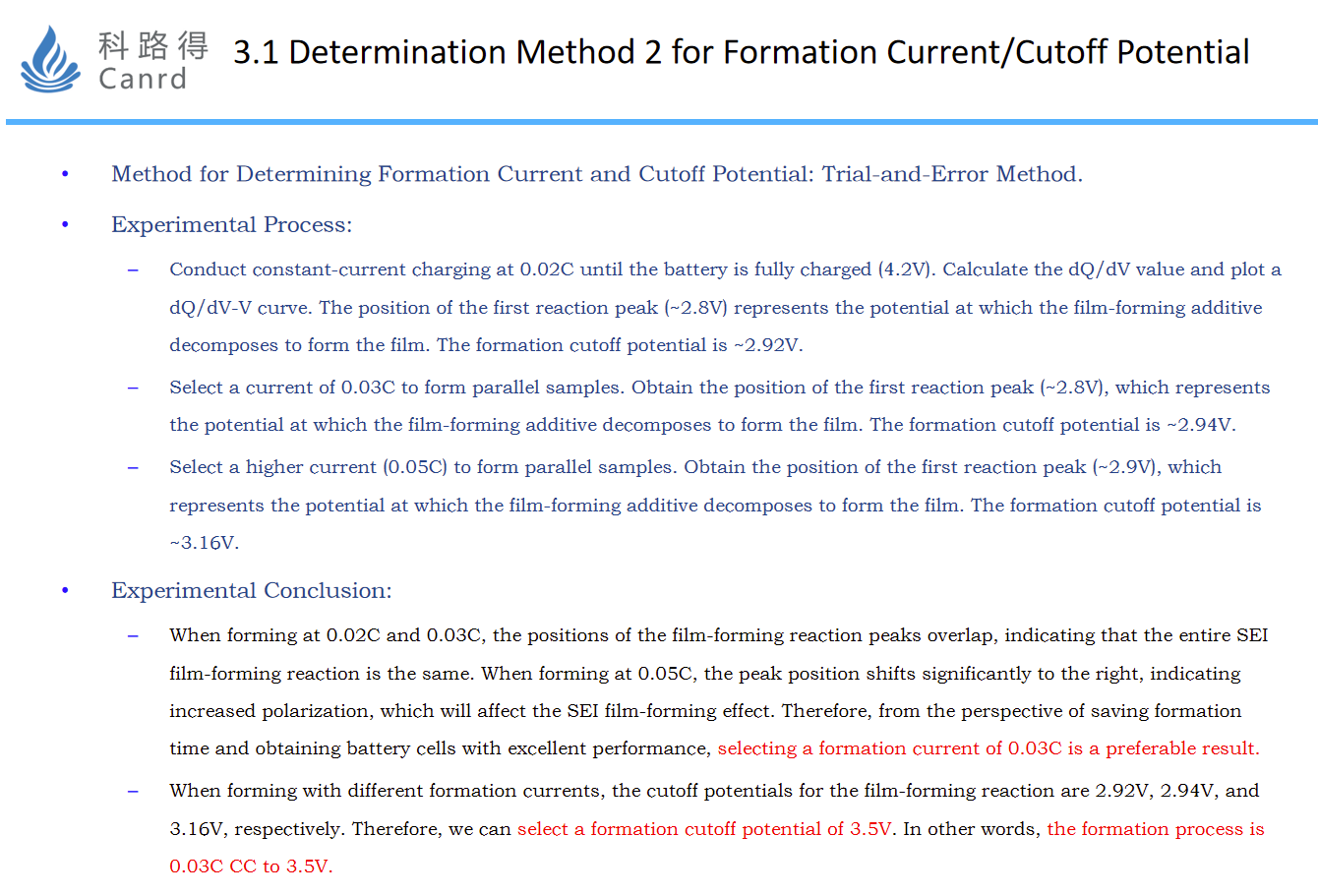
For the above analysis, as shown in the ppt, the recommended formation flow is: 0.03C to 3.5V; Of course, can directly do a full charge, that is, 0.03C to 4.2V.