Today, we will focus on the formation of the full battery. Formation is mainly to activate and passivate the cathode and anode electrode materials to prevent the active materials from continuing to react with the electrolyte. The formation process will be divided into two lectures, the first lecture: basic principles; Lecture 2: Process Parameters.

The first lecture: basic principles
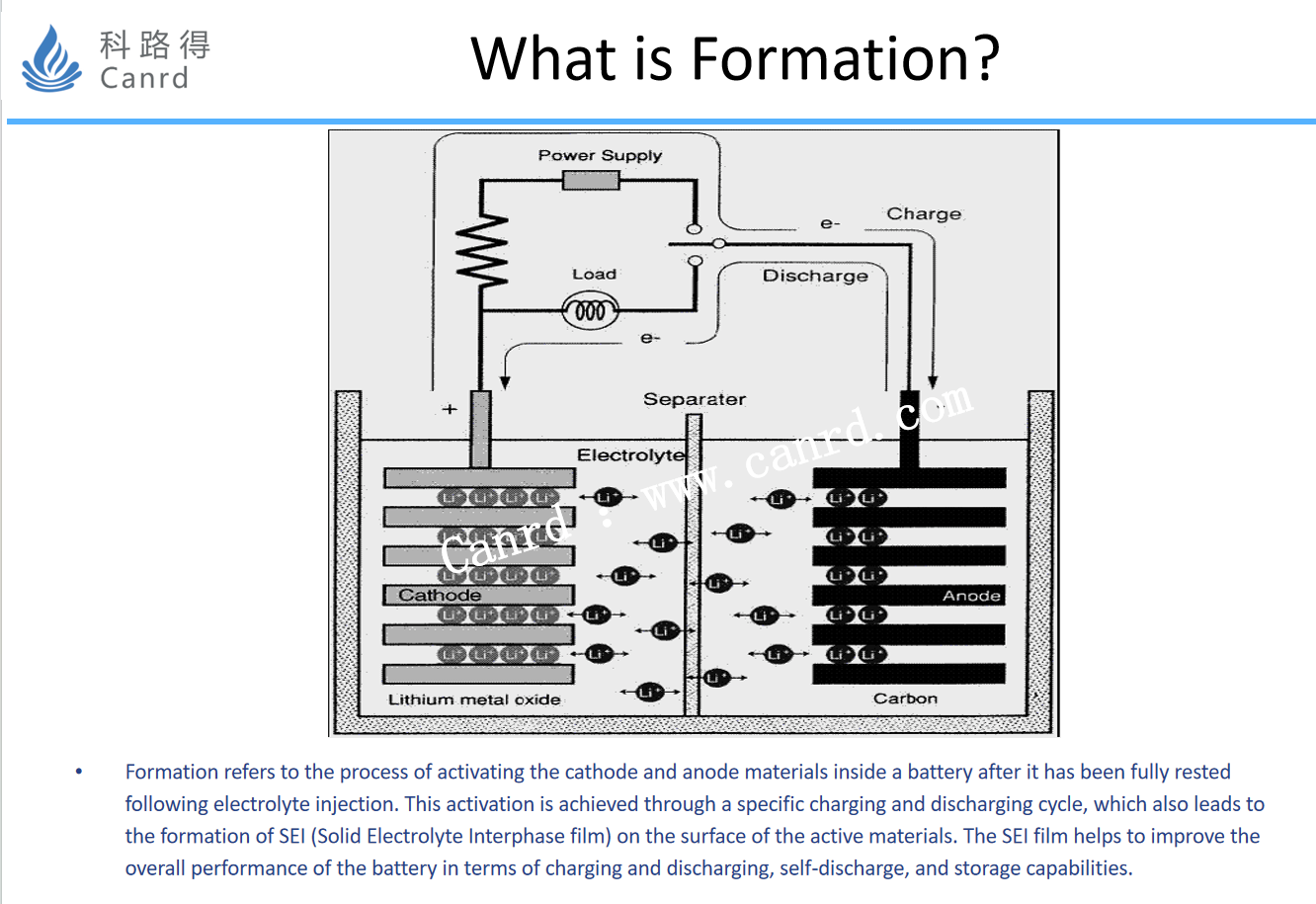
The basic concept of formation is the process by which a certain current activates a battery and forms SEI.
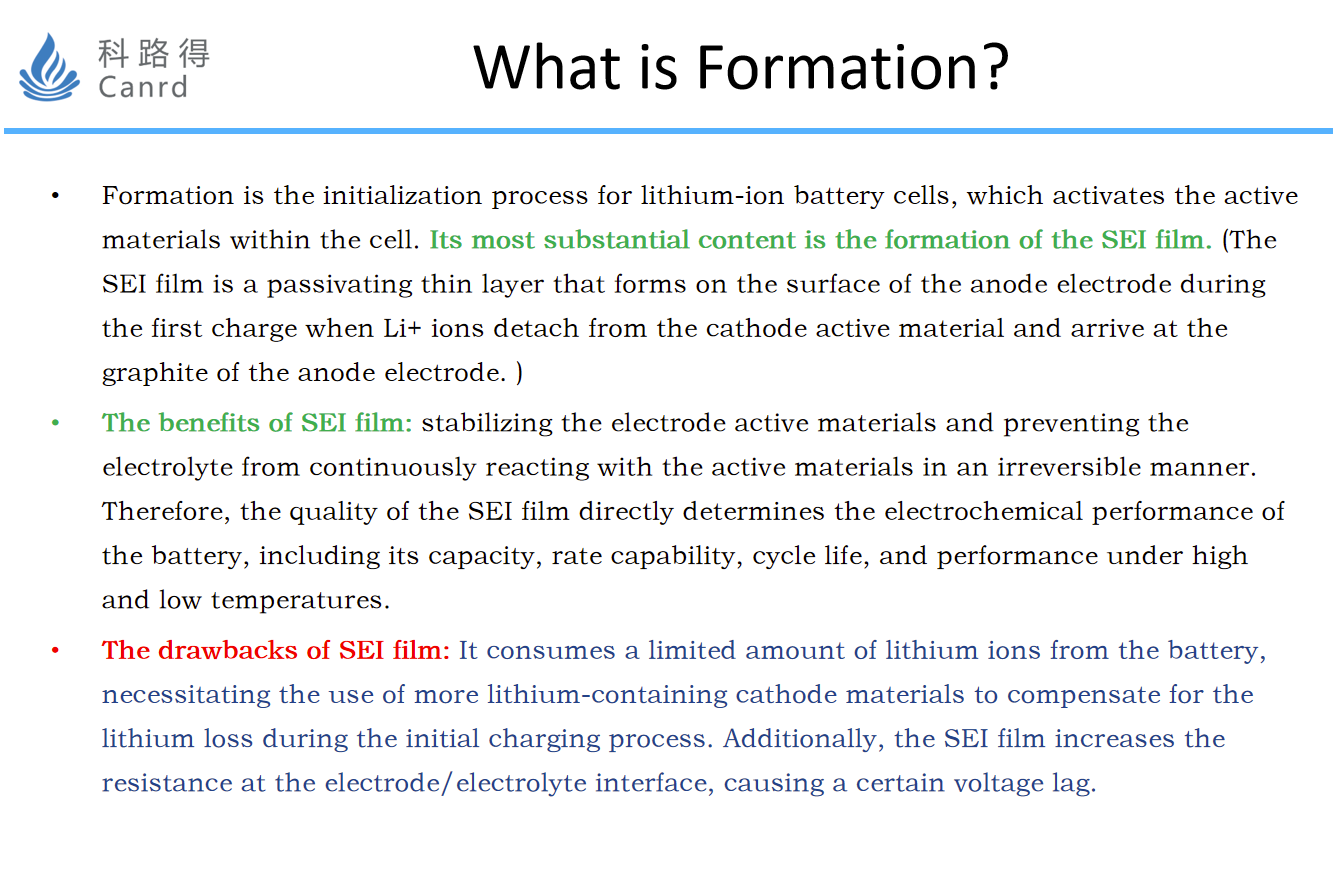
Therefore, the core research content is to study SEI. SEI has a huge impact on battery capacity, rate, cycle and other performance. However, at the same time, when SEI is formed, the limited lithium source in the battery will be consumed, and the first coulombic efficiency of the battery will be reduced, so the formation of SEI must be strictly controlled.
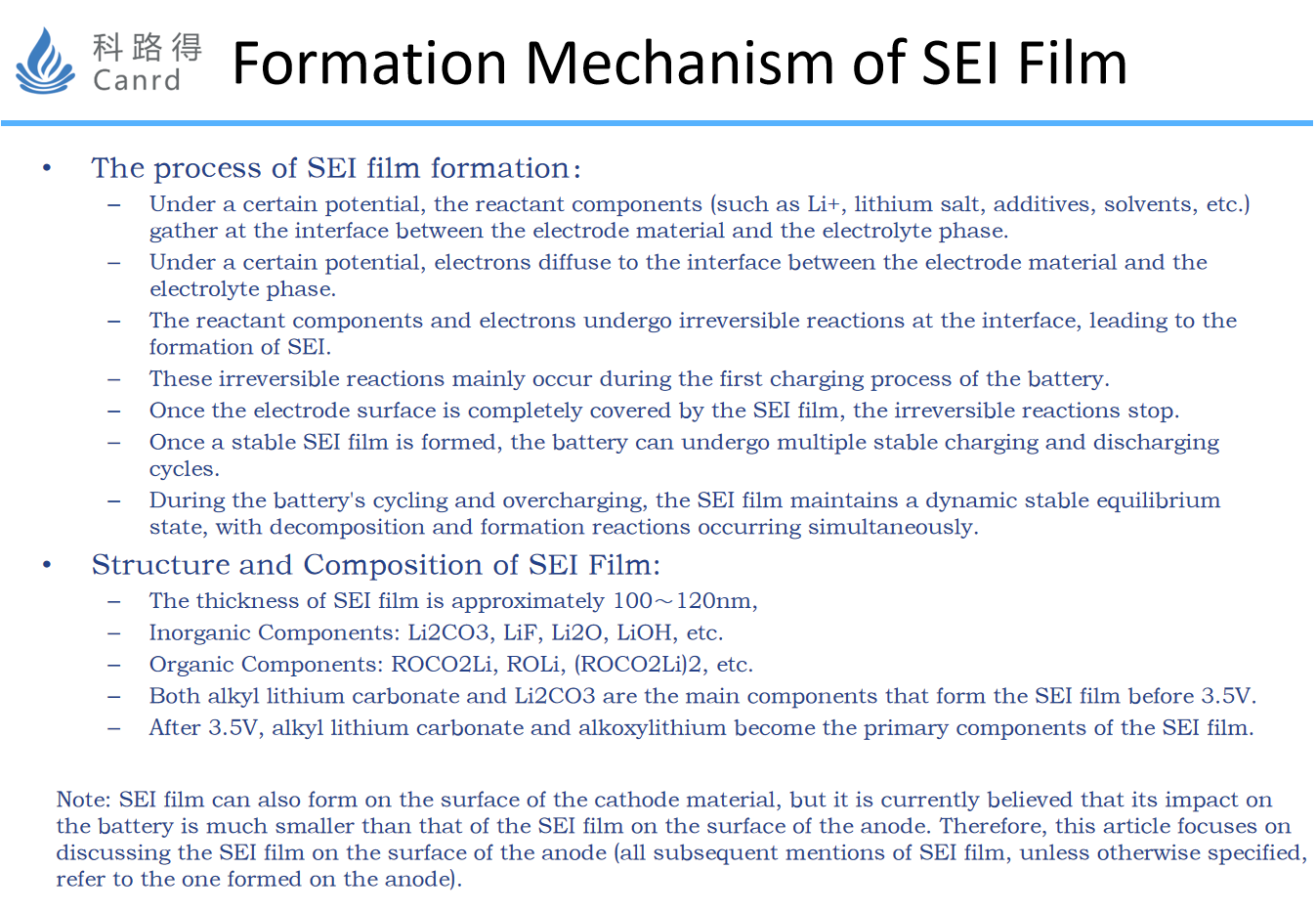
The film-forming process of SEI, as well as the specific components, are shown in the figure above. The above process and the composition of SEI are obtained through specific research: the formation of SEI is essentially a chemical reaction, so the whole reaction process can be deduced from the reactants, reaction products, etc. The reactants formed by SEI are mainly electrolytes, graphite, etc., and the products are mainly SEI and gas components (that is, a large amount of gas will be produced during the formation process).
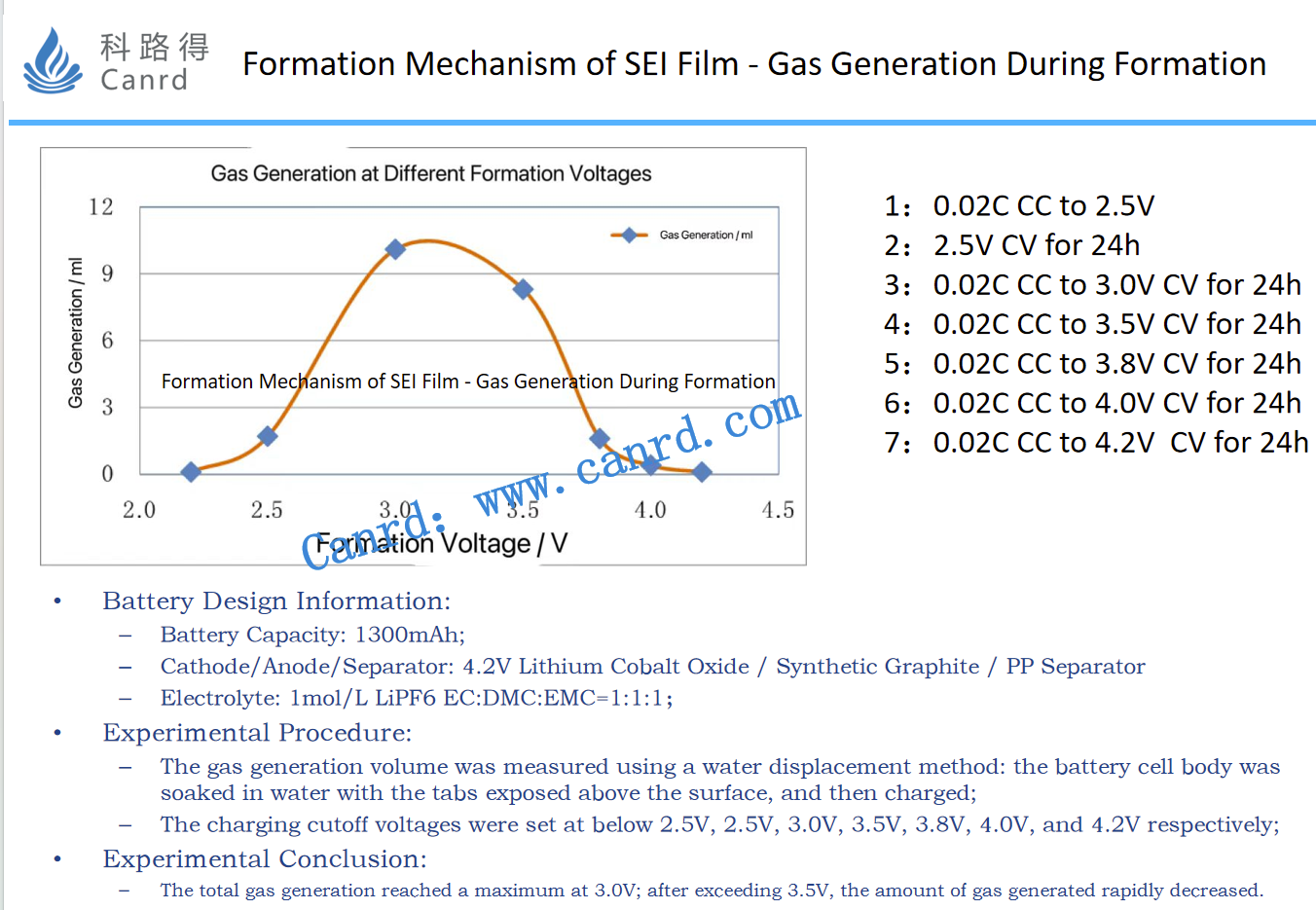
As can be seen from this diagram, the gas is not uniformly produced at the time of formation, and gas production is maximum at 3.0V. The specific method was obtained by testing using the drainage method.
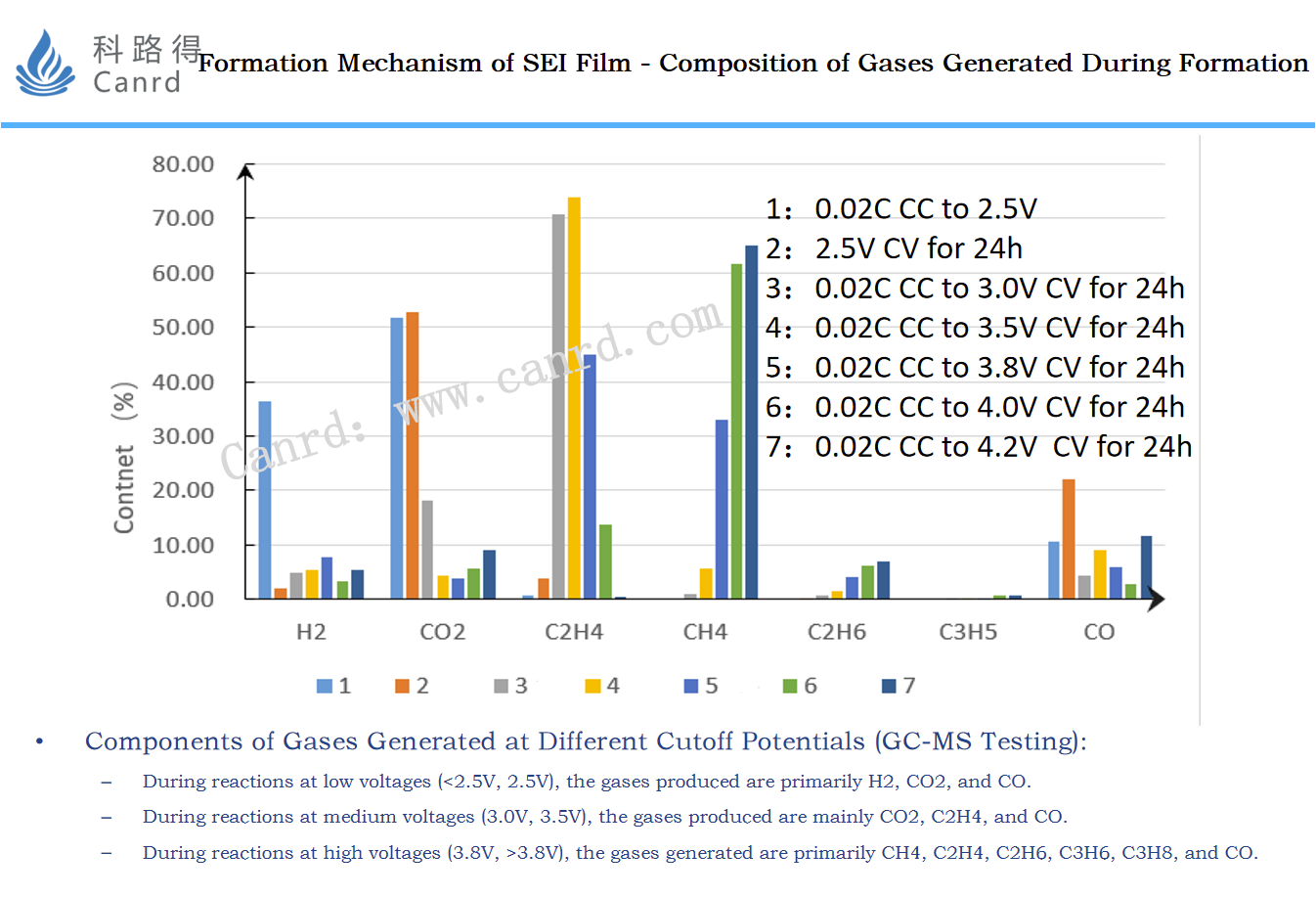
The tested the composition of the gas, it can be obtained that the composition ratio of the gas is different in different reaction stages. Of course, the main components include H2, CO2, CO, C2H4, CH4, C2H6, etc.

Combined with the previous two diagrams, the reaction process that is roughly deduced is the content of this diagram.
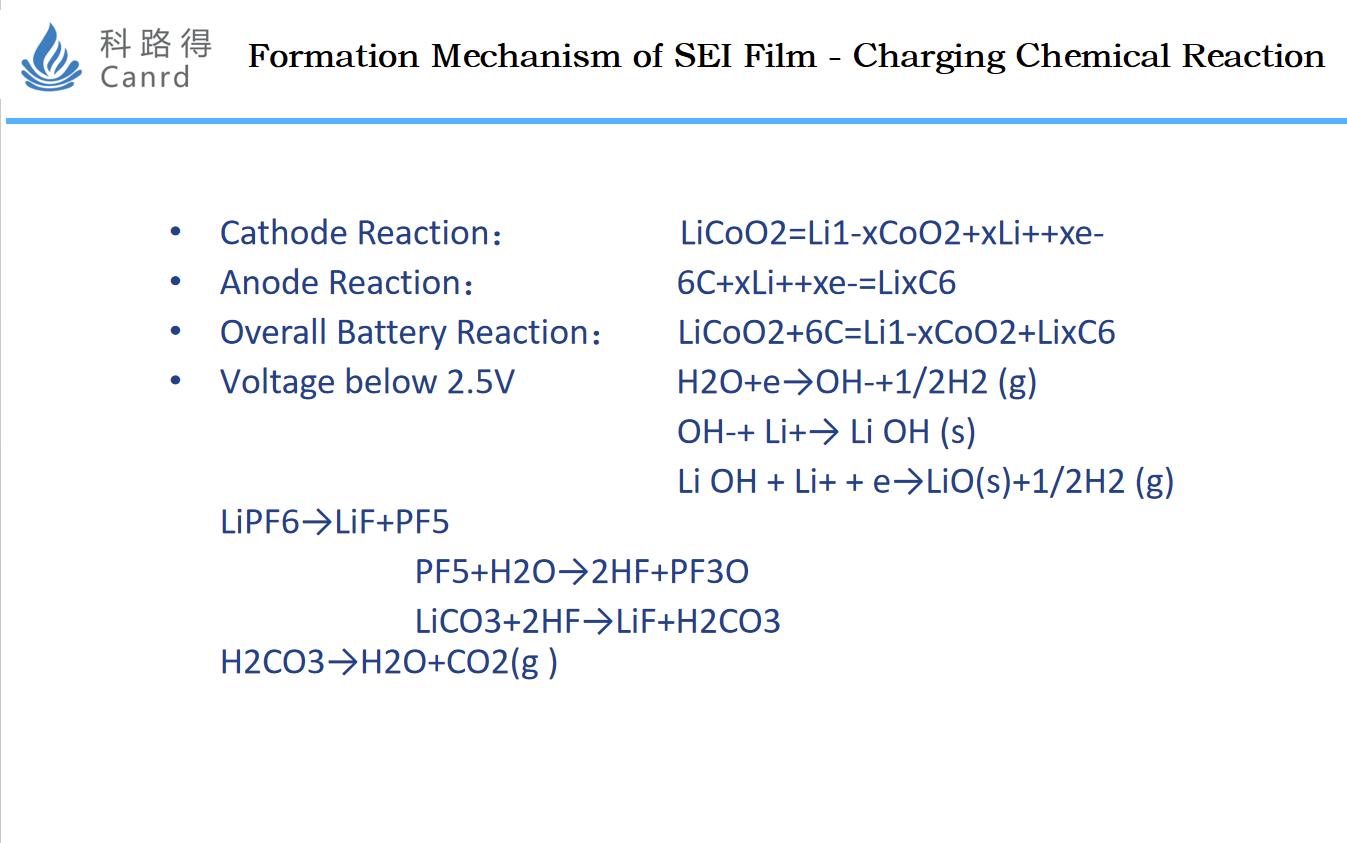
Summarized as a reaction equation:
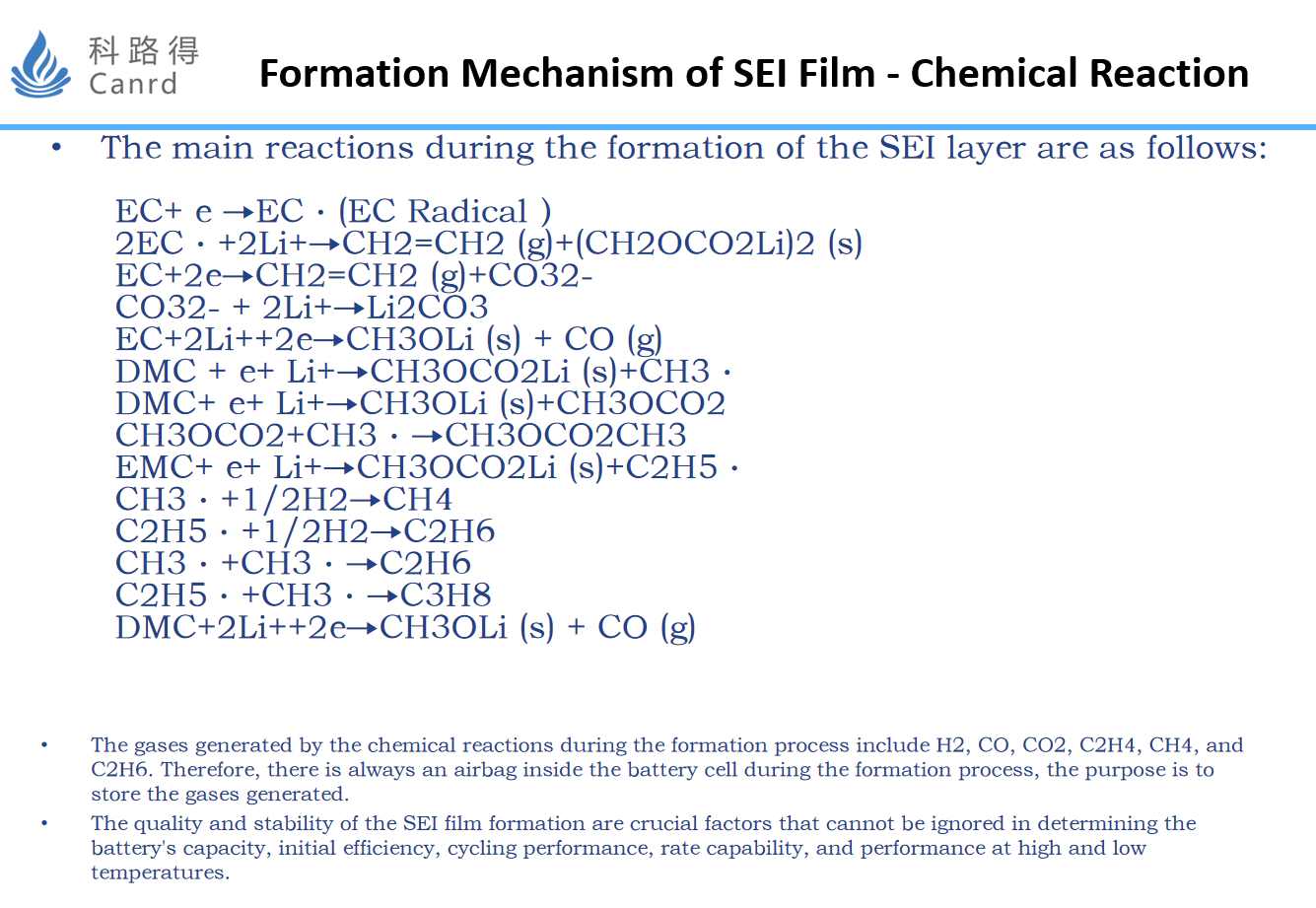
The above is based on the nature of the chemical reaction, and the reaction mechanism of the formation of SEI film is speculated.
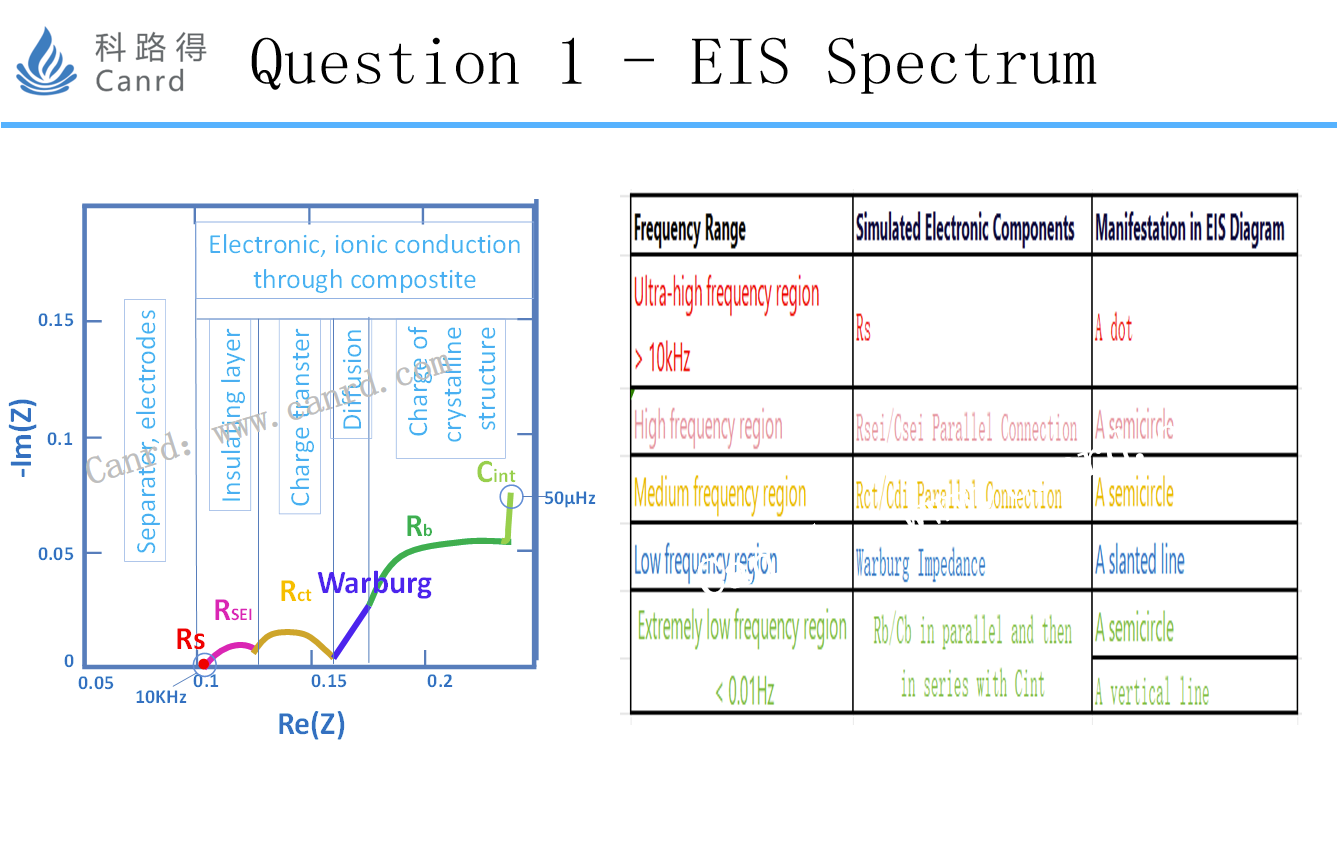
Shown on the left in the figure above is a standard EIS spectrum, divided into 5 sections.
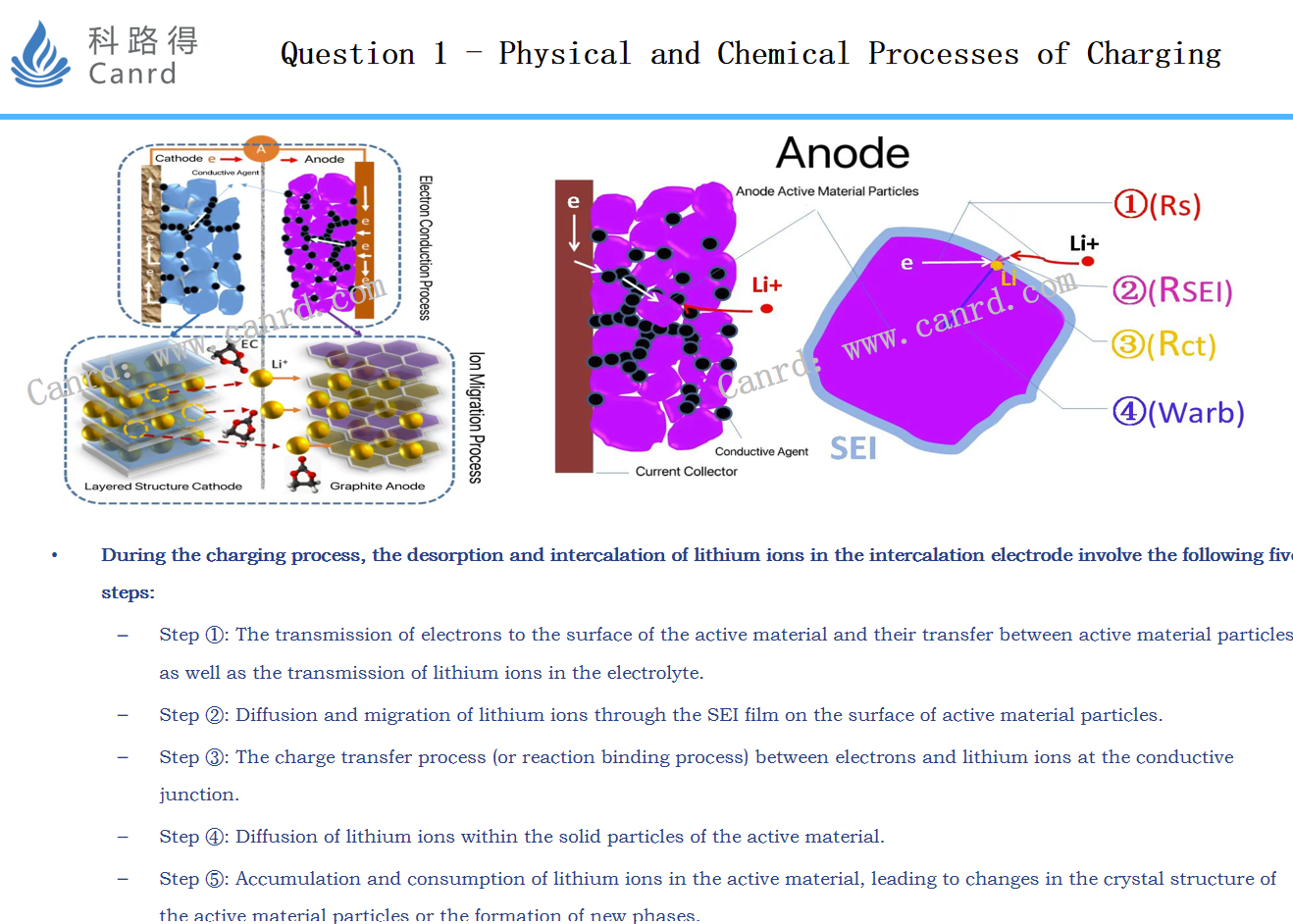
The physicochemical reaction steps in the whole charging process can be divided into these five steps. And these five steps correspond one-to-one to the five parts of the EIS map.
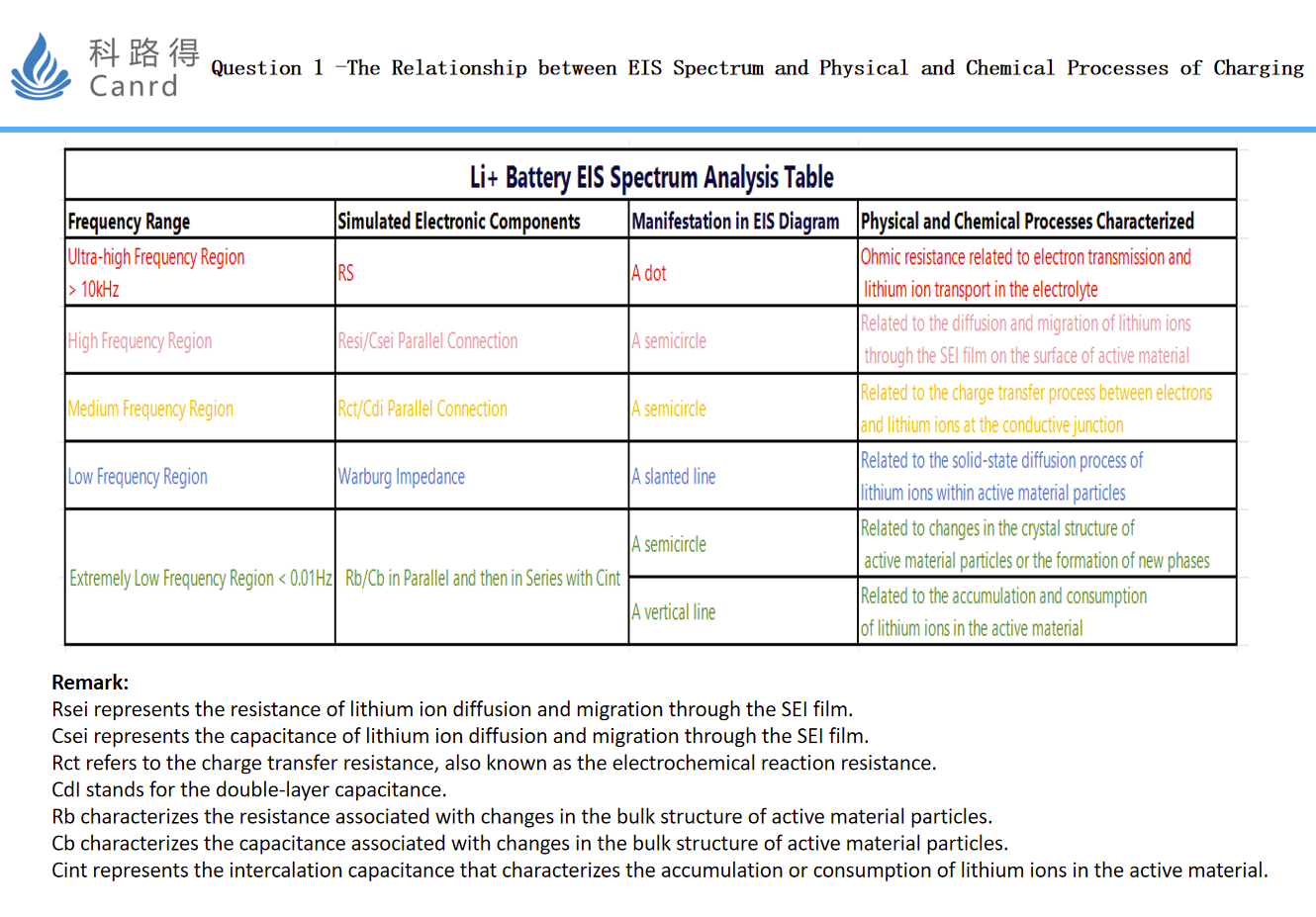
Of course, this diagram can only be measured by an ideal battery system (i.e., an EIS map with 5 parts that are very separated), and many times two or more EIS maps are overlapped together. The above three figures should be very helpful for everyone to have a preliminary understanding of the EIS map: it can correlate the physical and chemical reaction process with EIS.
One thing that needs to be corrected is that lithium is present as atoms inside the active particles.
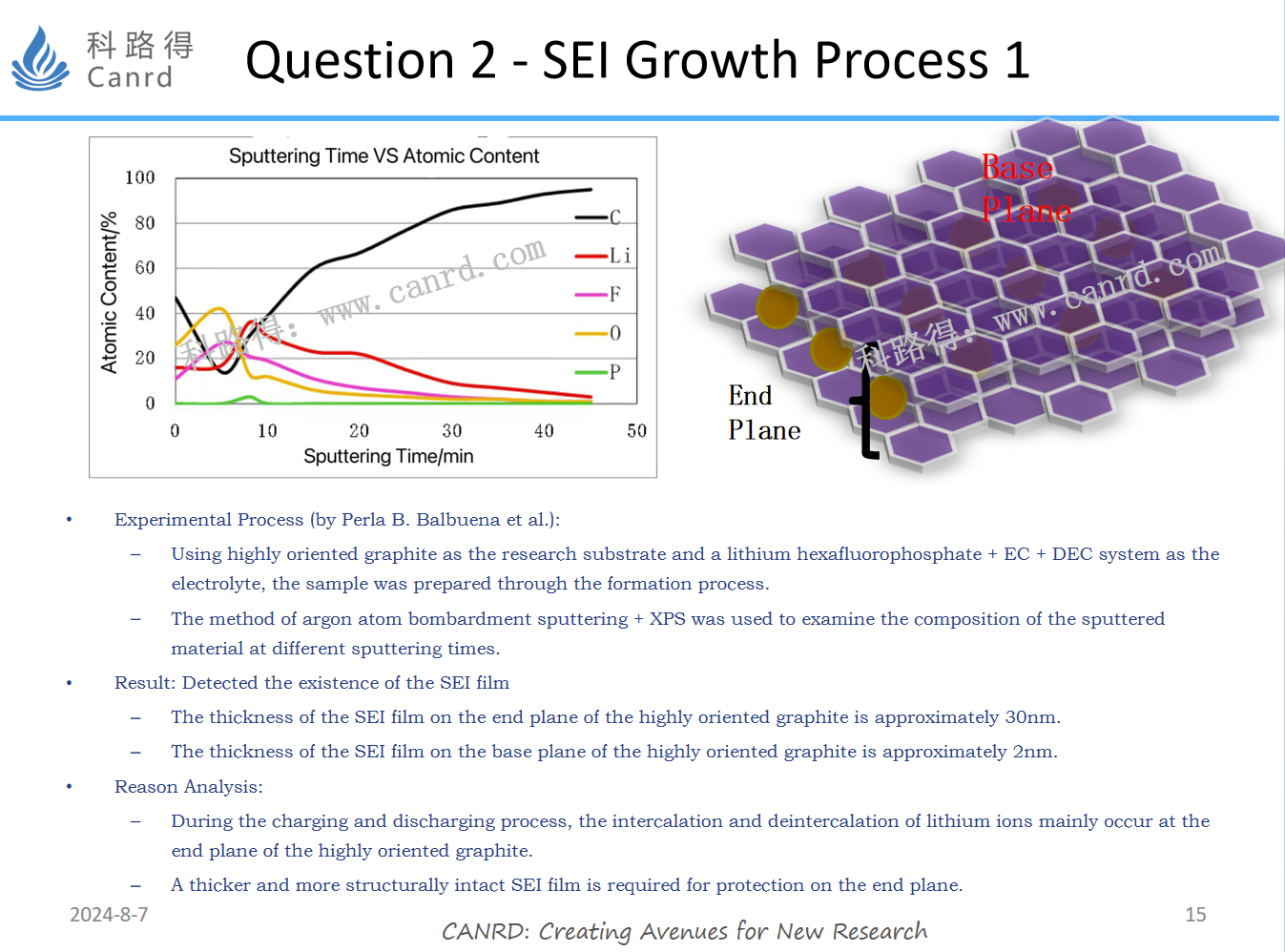
SEI is definitely testable. The specific experiment and test method are shown in the figure above, and highly directional graphite is used as the reactant. The argon atom bombardment sputtering + XPS test was used to test the material components sputtered at different bombardment times. Will the SEI film grow indefinitely along the thickness direction? The answer is no.
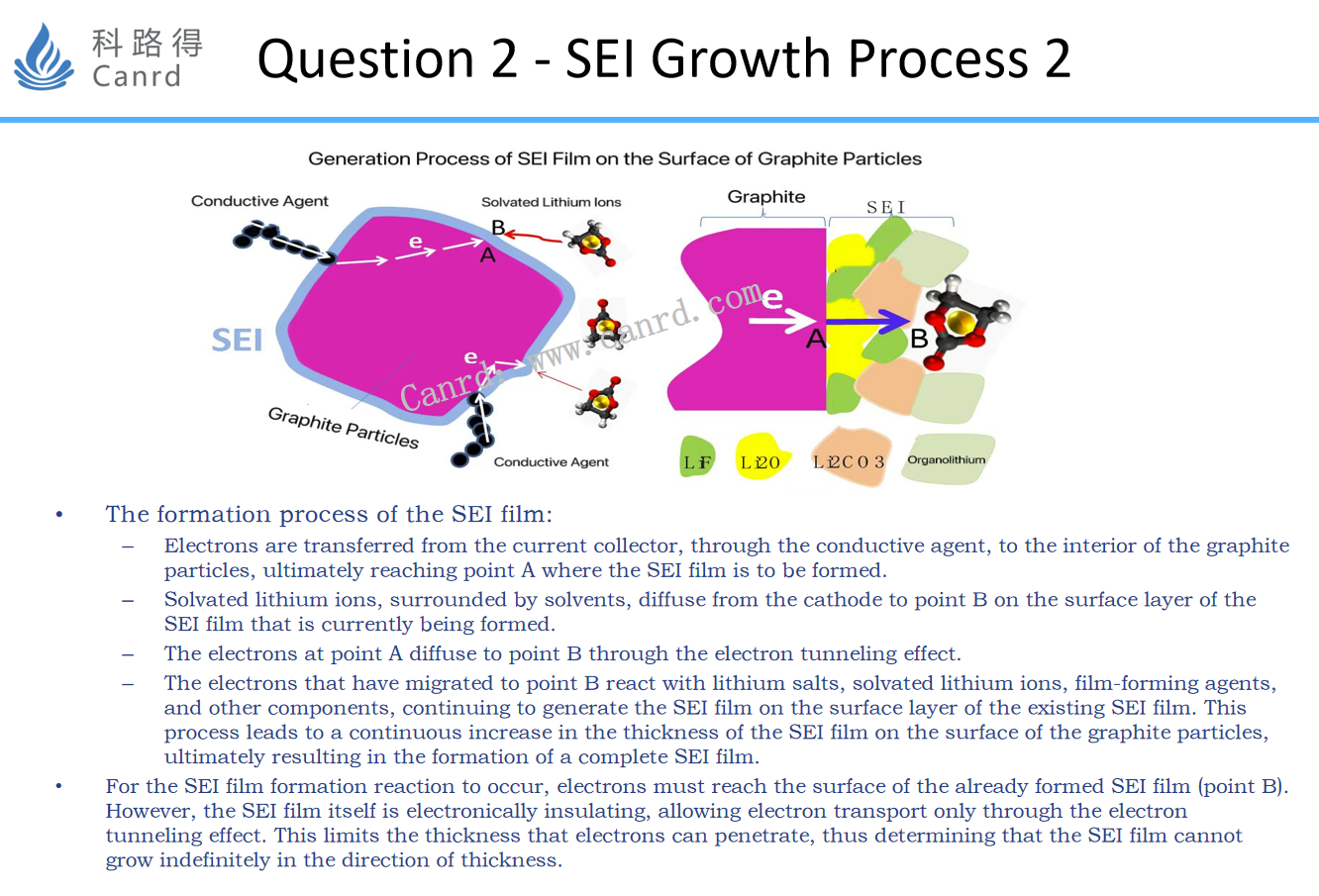
This diagram illustrates the growth process of SEI in detail. For SEI to grow, two conditions must be met:
1. Electronically arrive at point B;
2. The reactant reaches point B.
And as we all know, in order for an electron to reach point B, it must pass through point A and then penetrate SEI to reach point B, but the SEI film itself is insulated. Therefore, when its thickness reaches a certain value, electrons will not be able to reach point B from point A, and SEI will stop growing. The transition of electrons from point A to point B should be the result of the electron tunneling effect. The above two figures first mention a specific experimental method for testing SEI. Next, let's talk about the last question: Are the components of the SEI film consistent in the direction of thickness?
The formation of SEI is divided into single-electron reaction and two-electron reaction, and single-electron reaction forms organic SEI; The two-electron reaction forms inorganic SEI.
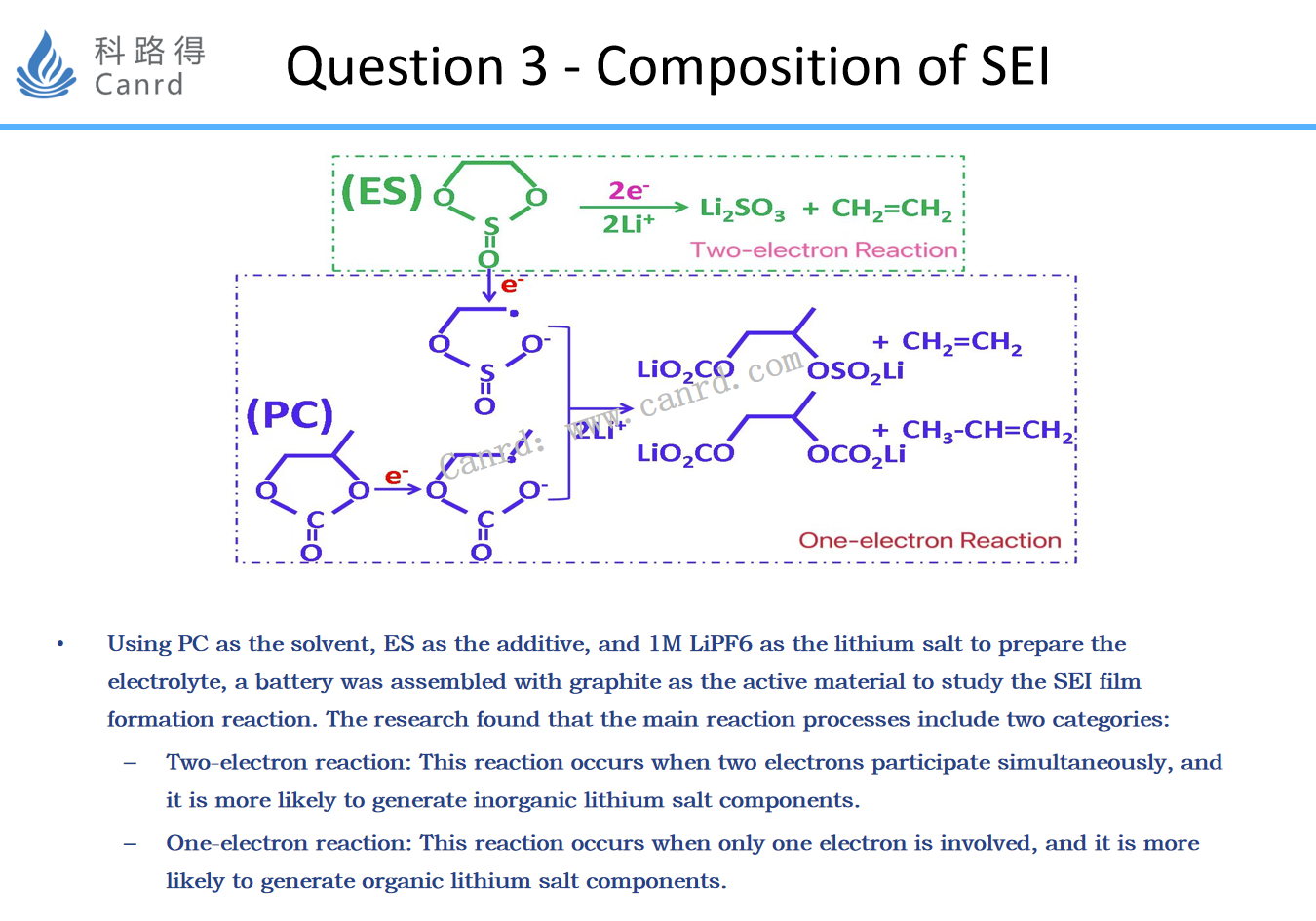
This is relatively easy to understand: inorganic molecules are small, the steric hindrance is small, and it is easier to accept double electrons; Whereas, organic matter has a large molecule and a large steric hindrance, so it is more inclined to accept single electrons.
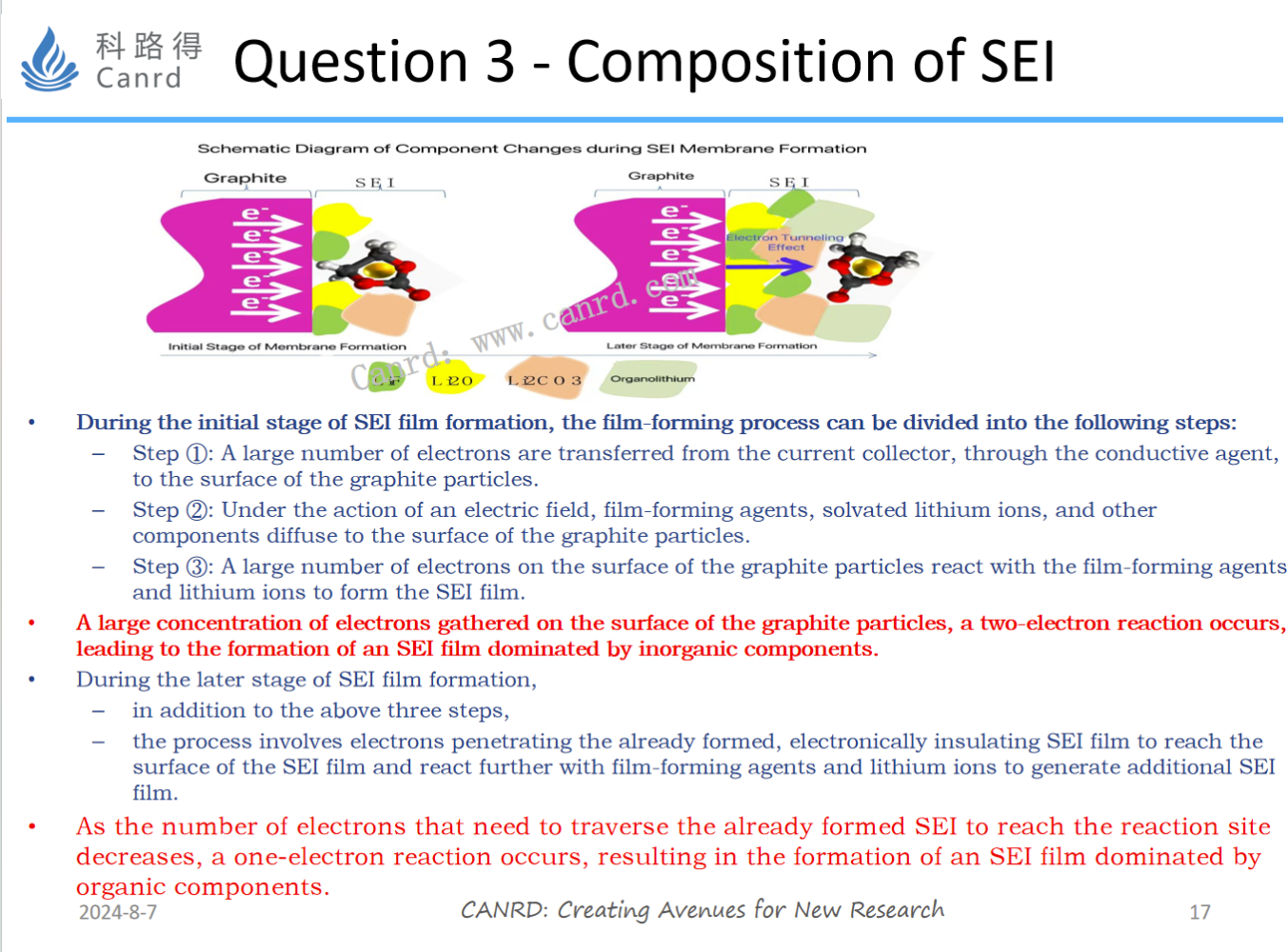
In Problem 2, we talked about the fact that the electrons at point B are transitioned from point A. Then when the SEI is thinner, the electrons can easily reach the B point. Therefore, if there are enough electrons at point B, it is easier for a two-electron reaction to occur. When the SEI is thicker, it is more difficult for the electrons to reach point B, and the resistance becomes larger, so fewer electrons reach point B, so it is easier for a single-electron reaction to occur. The SEI in which the internal components are inorganic components and the external components are organic components is also relatively ideal: the inorganic components have good barrier effect, but the toughness is poor; The organic component has poor barrier effect, but good toughness, and is more suitable for acting as a protective layer on the surface.
Through the above three questions, we have basically explored the key factors that affect the formation:
1. Electron concentration;
2. Reactant concentration.
These two factors are the key factors that affect the control of SEI film formation.