Lithium-ion full battery production process training-baking and liquid injection
Preface
The production of commercial full batteries is a complex system engineering, the core of which mainly includes three parts: material system development, battery cell structure design, and battery cell production control. To prepare a commercial full battery with excellent performance, you must master the above three core parts. After mastering the production of commercial full batteries, it will be extremely easy to assemble and produce button half batteries, button full batteries, and flexible batteries with simple structures (one positive electrode/one negative electrode stacked structure). In view of this, Dr. Ke Lude will continue to give lectures in the QQ group (group number: 308486834), and systematically introduce the commercial full battery production technology through live text and picture broadcasts, and each knowledge point is systematically introduced. All Ke fans are welcome to join the group to watch and communicate. In the last course, Dr. Ke mainly introduced the packaging process of making batteries and some problems and precautions encountered in the "Ke Lude Industrial Soft Pack Full Battery Production Technology Forum". Today, I will introduce the baking and injection of soft pack batteries, and I hope it will be helpful to everyone. This course is also presented in two parts.
Today we will mainly introduce the three processes of baking, filling and standing of the full battery. I hope that some of the relevant knowledge points will be helpful to you. First, let's introduce baking.
Baking
The purpose of baking is to remove moisture from the bare battery cells.
1. H,O will cause LiPF to decompose, resulting in an increase in HF: LiPF6 + HO -> LiF + POF3 + 2HF
2. H,O will react with the organic solvent in the electrolyte to generate alcohol and CO, such as: PC + H,0 = propylene glycol + CO,
3. H,O will decompose during the formation process to produce H, consume lithium ions, reduce the battery's initial efficiency and capacity, and damage the battery interface
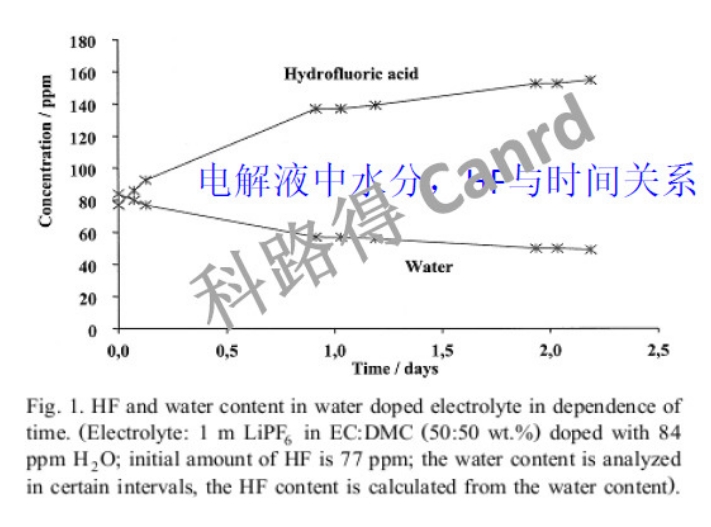
The moisture in the battery cell will affect the electrolyte, battery gas production, first effect, etc., and needs to be controlled.
The main purpose of baking is of course to remove moisture from the bare battery cell. This moisture is mainly in the positive electrode material, negative electrode material and separator. Why do we need to remove moisture? Dr. Ke mainly cited three influencing factors. Of course, we will also talk about it later. If the moisture is not well controlled, it will also affect the electrolyte injected in the injection process, resulting in the first problem as shown in the figure above, and the HF content in the electrolyte will increase. Some students may ask, at what level should it be controlled? Let's look down.
Baking
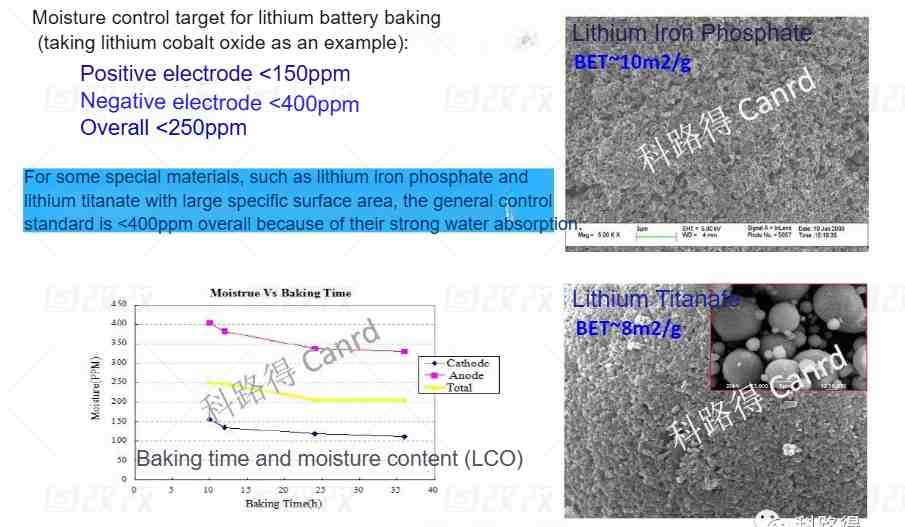
Because there are many chemical systems, we cite here a commercially mature material system, positive electrode lithium cobalt oxide + negative electrode graphite. The water content is tested by the Karl Fischer method. The overall water content needs to be controlled at 250ppm. However, because there are many types of materials, when we use lithium iron phosphate and lithium titanate as shown in the figure, the BET of the material is too large and the water absorption is relatively strong, so the overall control level is about 400ppm. I wonder if any students have noticed that the water content of the negative electrode is greater than that of the positive electrode. In fact, graphite itself is hydrophobic, but because it uses a water-based slurry and the positive electrode is an oil-based NMP system, the water content of the negative electrode is high. The picture in the lower left corner of the above picture is to illustrate that as the baking time increases, the water content can decrease, but it will reach a stable level. How to control the baking?
Bake
Three control factors for baking: temperature, vacuum and time
Increasing the temperature is a feasible method, but due to the poor heat resistance of the diaphragm in the battery cell, the general baking temperature of the battery cell is below 85°C. In order to improve efficiency, the baking time needs to be shortened, so increasing the vacuum degree becomes a feasible solution.

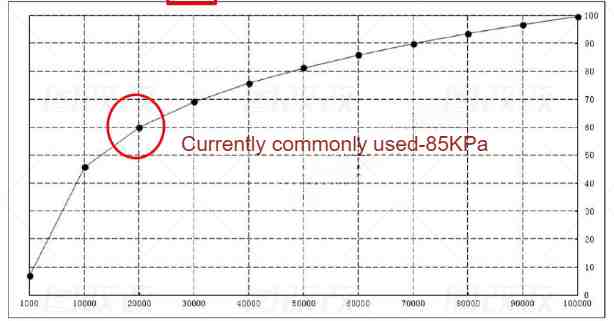
Here are three factors that affect baking: temperature, vacuum and time. Temperature is relatively easy to understand. Increasing the temperature can help the volatilization of water, but because the diaphragm has poor heat resistance, the temperature is not easy to be too high. From the table below, we can see the relationship between vacuum and the boiling point of water. By increasing the vacuum, the boiling point can be effectively lowered, thereby achieving the effect of rapid baking and dehydration, which is also a current development direction of ovens.
Baking
Three ways of heat transfer: heat conduction, heat radiation, and heat convection.
Conventional ovens can only transfer heat through heat radiation under vacuum conditions, while contact ovens can quickly transfer heat through heat conduction and heat radiation.

Next, let's look at the baking method, that is, heat transfer. There are also three influencing factors: heat conduction, heat radiation and heat convection. Our conventional oven baking time is relatively long, because under vacuum, only heat radiation is conducting heat. Now the better baking is to use a contact oven. In addition to heat radiation, heat can also be conducted through the contact hot plate, which heats up much faster and greatly shortens the overall baking time.
Water content test
Test methods for water content
Karl Fischer method is a titrimetric method for quantitative determination of water content in liquids and solids. Karl Fischer titration can be applied in various fields, such as determining the water content in food, chemical reagents, pharmaceuticals, cosmetics or mineral oils. When determining the water content, sulfur dioxide and water react with iodine:
2 H20+S02+|>SO42-+21+4 H*
Adding alcohol (e.g. methanol, ethanol) to sulfur dioxide generates an acidic lipid in the pre-reaction, which is then neutralized by a base (e.g. imidazole, hereinafter referred to as "RN"):
CH,OH + SO,+ RN→(RNH)·(CH,OSO,)
Alkyl sulfite anions are oxidized to alkyl sulfates by iodine in the presence of water. At the same time, the brown-yellow iodine is decomposed into colorless iodide:
(RNH)·(CH,OSO,) + |,+ H2O + 2 RN ->(RNH)·(CH3OSO3)+ 2 (RNH)I

The reaction continues until all the water has been consumed and free iodine is detected in the titration solution. The endpoint is indicated by a double voltage measurement, i.e. the potential on the polarized double platinum (needle) electrode drops below a certain value (e.g. 100 mV).
Just now I mentioned that baking is to remove moisture, and also mentioned some control standards. Have you ever thought about how to test moisture? So now I will introduce the test method of moisture.
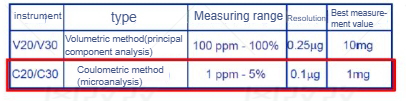
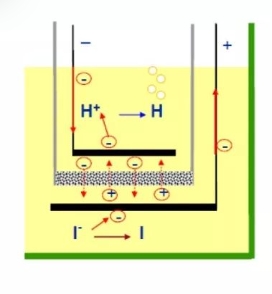
At present, the internationally accepted method for trace moisture is the Karl Fischer moisture test method. The basic mainstream is to use the tester of Mettler and Swiss Metrohm, so everyone's test results can be compared horizontally. I won't go into details about the basic principle. Simply put, the electrode is heated to let the moisture escape, and then it is sent to the reaction pool through the carrier gas. Water and iodine react, and the moisture content is calculated by detecting the change of iodine.
Water content test
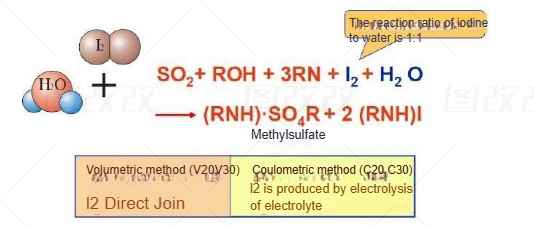
The Karl Fischer method is a recognized moisture testing method (commonly used in the lithium battery industry)
Methanol not only acts as a solvent, but also directly participates in the reaction, so the amount of methanol in the solvent must be greater than 50%
Faraday's law:
To produce 1 mol of chemical substance (single electron), 96485.309℃ of electricity is required 1 mol, = 2*96485.309 C1 mol, = 1 mol H2O
18,000 mg H20=1molH20=1 mol l2=2*96485.309C1 mg H20 = 10.72 C
(1C=1A*1s)
This is a supplement. It can be seen that the reaction amount of water and elemental iodine is 1 mol, and elemental iodine is produced from iodide ions by electrolysis. Therefore, the amount of elemental iodine can be known by recording the amount of electricity. The amount of elemental iodine corresponds to that of water, so the relationship between the amount of electricity and the water content is obtained.
Injection
Quality requirements of CLUDE electrolyte:
The whole process is configured in a glove box, H20<0.1ppm
Use high-purity salt and high-purity solvent (main solvent purity>99.99%)
Each sample configuration implements incoming material inspection + process inspection + shipment inspection to ensure that the quality meets the requirements
Shipment sample control standards: Density: standard value ±0.003g/cm3; moisture <20ppm; HF <50ppm
Special sealed aluminum bottles, vacuum packaging, to ensure that the electrolyte is not affected by external moisture during transportation and storage
Professional testing equipment: Mettler moisture tester, pH meter, titrator, density meter
In addition to the positive electrode, negative electrode, separator and other materials in the bare battery cell, the moisture content in the electrolyte also needs to be strictly controlled (moisture content <20ppm).
After the battery is baked and cooled to room temperature, it will be injected. The electrolyte here at CROOD has strict quality requirements, because as mentioned earlier, moisture has an impact on the performance of the battery. So basically the electrolyte needs to be prepared in a glove box, and the moisture standard is <20ppm. So how much electrolyte is needed for injection and how to determine the injection amount, let's take a look.
Injection
Selection of injection amount
1) First, the theoretical demand for electrolyte (i.e. the minimum amount of electrolyte) can be estimated based on the true density, porosity and compacted density of the material
2) On this basis, according to the size of the battery, the application requirements (such as rate type, energy type, etc.), and a certain amount of surplus, the common injection coefficient of injection amount is obtained: lithium cobalt oxide system ~2.0g/Ah, ternary system 3.0g/Ah
The injection process is usually completed in a glove box, and the relative humidity is controlled to <1%.
Taking the soft-pack battery as an example, the injected electrolyte needs to completely immerse the bare battery to ensure that the electrode is fully wetted.
First, some calculations are needed to get the theoretical minimum amount of liquid injection, and then some adjustments are made according to customer needs. Finally, it is based on some experience and some margin. So it is a value of calculation + experience. This value is related to materials, design, and application. I can only teach you one method. Taking soft-pack batteries as an example, the electrolyte must basically soak the bare battery to ensure that the entire electrolyte is fully infiltrated. The injection process must be completed in a low-humidity room because there are bare batteries and electrolytes. This is very important.
Stand
Purpose of static
1) The main purpose of static is to allow the electrolyte to fully penetrate into the inside of the pole piece and the pores of the diaphragm
2) In order to accelerate the infiltration, the static process is in a vacuum state, and the electrolyte infiltration is accelerated
3) Since the electrolyte can only infiltrate from the head and tail of the wound battery, a certain temperature and time are required to ensure the infiltration effect (if the battery is thick and long, the infiltration conditions need to be optimized)
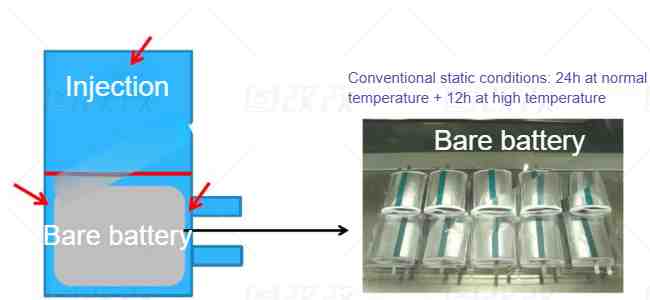
The last one is the static process. Different from universities, the industry will use vacuum pumping. The main purpose is to extract the air in the aluminum-plastic film bag to allow the electrolyte to contact the electrode, and to remove the air in the pores of the electrode to facilitate the rapid infiltration of the electrolyte. At the same time, because the bare battery cell is rolled up and there is pressure between the layers, it is also necessary to achieve sufficient infiltration through high temperature and long time, generally 36 hours. Because the button battery in universities is a single piece, the amount of electrolyte is sufficient and the compaction density is not large, so the infiltration time is often only a few hours.
Moisture influence
Effect of moisture on battery performance:
1. The H20 in the battery will react as follows at the negative electrode during the first charge:
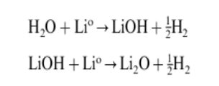 On the one hand, this process consumes active L, reducing the cycle efficiency of the battery; on the other hand, the H2 generated by the reaction will increase the internal pressure of the battery, affecting the consistency of the first charging current distribution. The LiOH and Li20 generated by the above reaction will eventually react with HF to generate LiF deposited on the negative electrode surface.
On the one hand, this process consumes active L, reducing the cycle efficiency of the battery; on the other hand, the H2 generated by the reaction will increase the internal pressure of the battery, affecting the consistency of the first charging current distribution. The LiOH and Li20 generated by the above reaction will eventually react with HF to generate LiF deposited on the negative electrode surface.
2. During the first charge, the solvent will be reduced at the negative electrode and the active L will be consumed:
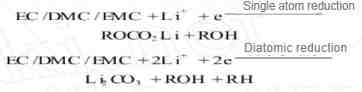
The alkyl lithium carbonate generated by the single electron reduction process can also react with trace amounts of water in the electrolyte:
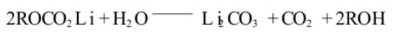
When C02 is generated, the reaction continues on the negative electrode surface: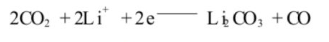
LiF and Li2CO3 are the main components of SEI, so a moderate amount of water (below 150ppm) helps to form a stable, uniform and dense SEI film mainly composed of Li2c03. When the SEI film completely covers the negative electrode, the irreversible reaction stops immediately. After SEI is formed, if there is still water in the battery (the water content is above 150ppm), H20 will continue to consume active L and cause LiPF6 to decompose, resulting in battery bloating, increased internal resistance, and SEI will also dissolve, causing performance deterioration.
HF Impact
Effect of HF on battery performance:
HF in the electrolyte generally undergoes the following reactions:
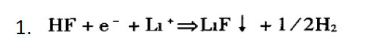
The LF generated by this reaction has good thermal stability, but its conductivity is poor, which will increase the impedance of the electrode/electrolyte interface and increase the internal resistance of the battery:
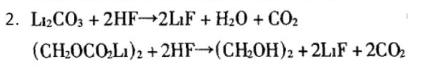
HF will dissolve the SEI film, that is, react with L2C03 in SEI to generate LF. This not only destroys the stability and density of SEI, but also increases the interface impedance of the electrode; in addition, the H20 and alcohols generated by this reaction will in turn promote the hydrolysis of LPF6 to generate HF. This process is repeated continuously, resulting in a continuous decrease in battery capacity and cycle efficiency, and ultimately serious damage to performance.
3. In addition, HF in the electrolyte reacts with the oxide positive electrode material, causing the metal ions in the positive electrode material to dissolve. For example, Mn ions are easily dissolved in the electrolyte of LiMn2O4 in LiPF6, resulting in its performance degradation:
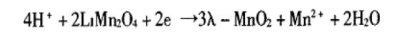
HF has three main effects. On the one hand, the generated LiF is a component of SEI, and the internal resistance will increase. In addition, it will dissolve SEI, which will affect the stability and density of SEI and make the cycle worse. Finally, for some positive electrode materials, it will cause metal ions to dissolve, resulting in performance deterioration. So the message that today's class wants to convey is to strictly control the water content in the materials and electrolytes, so as to obtain a battery with excellent performance. SEI will also be introduced in the next class, the formation process, so stay tuned. Our course ends here, thank you!